Related Research Articles

A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.

William Nunn Lipscomb Jr. was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry.

X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information.
Cubane is a synthetic hydrocarbon compound with the formula C8H8. It consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.
In chemistry, a hypervalent molecule is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride, sulfur hexafluoride, chlorine trifluoride, the chlorite ion in chlorous acid and the triiodide ion are examples of hypervalent molecules.

Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.
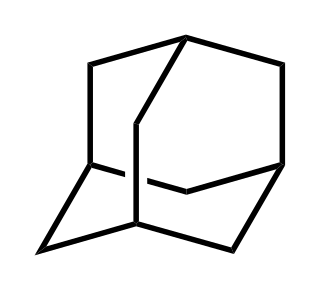
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.

Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons.
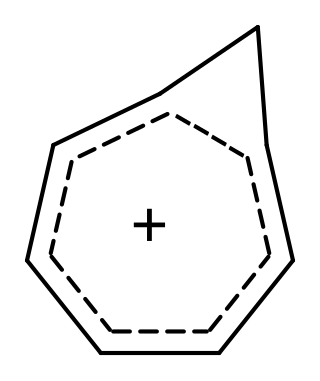
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element neon, which was shown by mass spectrometry to have at least two stable isotopes: 20Ne and 22Ne. Mass spectrometers were used in the Manhattan Project for the separation of isotopes of uranium necessary to create the atomic bomb.

A unimolecular rectifier is a single organic molecule which functions as a rectifier of electric current. The idea was first proposed in 1974 by Arieh Aviram, then at IBM, and Mark Ratner, then at New York University. Their publication was the first serious and concrete theoretical proposal in the new field of molecular electronics (UE). Based on the mesomeric effect of certain chemical compounds on organic molecules, a molecular rectifier was built by simulating the pn junction with the help of chemical compounds.

Harry Barkus Gray is the Arnold O. Beckman Professor of Chemistry at California Institute of Technology.
In chemistry, a halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bond, the result is not a formal chemical bond, but rather a strong electrostatic attraction. Mathematically, the interaction can be decomposed in two terms: one describing an electrostatic, orbital-mixing charge-transfer and another describing electron-cloud dispersion. Halogen bonds find application in supramolecular chemistry; drug design and biochemistry; crystal engineering and liquid crystals; and organic catalysis.
Jay Richmond Winkler, Ph.D. is an American physical chemist, currently director of the Beckman Institute Laser Resource Center at the California Institute of Technology. He has authored over two hundred twenty five articles on applications of inorganic spectroscopy, including the pioneering study of intramolecular electron transfer reactions in biological systems.
Nuclear magnetic resonance crystallography is a method which utilizes primarily NMR spectroscopy to determine the structure of solid materials on the atomic scale. Thus, solid-state NMR spectroscopy would be used primarily, possibly supplemented by quantum chemistry calculations, powder diffraction etc. If suitable crystals can be grown, any crystallographic method would generally be preferred to determine the crystal structure comprising in case of organic compounds the molecular structures and molecular packing. The main interest in NMR crystallography is in microcrystalline materials which are amenable to this method but not to X-ray, neutron and electron diffraction. This is largely because interactions of comparably short range are measured in NMR crystallography.

Eluvathingal Devassy Jemmis is a professor of theoretical chemistry at the Indian Institute of Science, Bangalore, India. He was the founding director of Indian Institute of Science Education and Research, Thiruvananthapuram (IISER-TVM). His primary area of research is applied theoretical chemistry with emphasis on structure, bonding and reactivity, across the periodic table of the elements. Apart from many of his contributions to applied theoretical chemistry, an equivalent of the structural chemistry of carbon, as exemplified by the Huckel 4n+2 Rule, benzenoid aromatics and graphite, and tetrahedral carbon and diamond, is brought in the structural chemistry of boron by the Jemmis mno rules which relates polyhedral and macropolyhedral boranes to allotropes of boron and boron-rich solids. He has been awarded Padma Shri in Science and Engineering category by the Government of India.
Weitao Yang is a Chinese-born American chemist who is the Philip Handler Professor of Chemistry at Duke University. His main contributions to chemistry include density functional theory development, and its applications to chemistry.
Robert Tycko is an American biophysicist whose research primarily involves solid state NMR, including the development of new methods and applications to various areas of physics, chemistry, and biology. He is a member of the Laboratory of Chemical Physics in the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health in Bethesda, Maryland, USA. He was formerly a member of the Physical Chemistry Research and Materials Chemistry Research departments of AT&T Bell Labs in Murray Hill, New Jersey. His work has contributed to our understanding of geometric phases in spectroscopy, physical properties of fullerenes, skyrmions in 2D electron systems, protein folding, and amyloid fibrils associated with Alzheimer’s disease and prions.

Stephen L. Craig is the William T. Miller Professor of Chemistry at Duke University. He is the director of the Center for Molecularly Optimized Networks, a NSF Center for Chemical Innovation.
Michael J. Therien is the William R. Kenan, Jr. Professor of Chemistry at Duke University.
References
- ↑ "Duke Chemistry". Duke University Chemistry. Retrieved 27 June 2019.
- ↑ "Duke Physics". Duke University Physics. Retrieved 27 June 2019.
- ↑ "Duke Biochemistry". Duke University Biochemistry. Archived from the original on 10 July 2019. Retrieved 27 June 2019.
- ↑ "Award Abstract 1925690" . Retrieved 17 July 2019.
- ↑ "Academic Family Tree – John Hopfield". Neurotree - Hopfield. Retrieved 9 July 2019.
- 1 2 3 4 "Guggenheim Fellow Biosketch". Guggenheim Fellows. Retrieved 9 July 2019.
- ↑ Beratan, Betts, Onuchic (1991). "Protein electron transfer rates set by the bridging secondary and teriary structure". Science. 252 (5010): 1285–1288. Bibcode:1991Sci...252.1285B. doi:10.1126/science.1656523. PMID 1656523.
- ↑ Marder, Beratan, Cheng (1991). "Approaches for Optimizing the First Electronic Hyperpolarizability of Conjugated Organic Molecules". Science. 252 (5002): 103–106. Bibcode:1991Sci...252..103M. doi:10.1126/science.252.5002.103. PMID 17739081. S2CID 39158497.
- ↑ Priyadarshy, Risser, Beratan (1996). "DNA is not a molecular wire: protein-like electron-transfer predicted in an extended pi-electron system". J. Phys. Chem. 100 (44): 17678–17682. doi:10.1021/jp961731h.
- ↑ Kuhn, Beratan (1996). "Inverse Strategies for Molecular Design". J. Am. Chem. Soc. 100 (25): 10595–10599. doi:10.1021/jp960518i.
- ↑ Kondru, Wipf, Beratan (1998). "Theory-Assisted Determination of Absolute Stereochemistry for Complex Natural Products via Computation of Molar Rotation Angles". J. Am. Chem. Soc. 120 (9): 2204–2205. doi:10.1021/ja973690o.
- ↑ Zhang, Liu, Balaeff, Skourtis, Beratan (2014). "Biological charge transfer via flickering resonance". PNAS. 111 (28): 10049–10054. Bibcode:2014PNAS..11110049Z. doi: 10.1073/pnas.1316519111 . PMC 4104919 . PMID 24965367.
- ↑ Skourtis, Waldeck, Beratan (2004). "Inelastic electron tunneling erases coupling-pathway interferences". J. Phys. Chem. B. 108 (40): 15511–15518. doi:10.1021/jp0485340.
- ↑ Wang, Hu, Beratan, Yang (2006). "Designing molecules by optimizing potentials". J. Am. Chem. Soc. 128 (10): 3228–3232. doi:10.1021/ja0572046. PMID 16522103.
- ↑ Virshup, Contreras-Garcia, Wipf, Yang, Beratan (2013). "Stochastic voyages into uncharted chemical space produce a representative library of all possible drug-like compounds". J. Am. Chem. Soc. 135 (19): 7296–7203. doi:10.1021/ja401184g. PMC 3670418 . PMID 23548177.
- ↑ "Beratan Lab".
- ↑ "Google Scholar" . Retrieved 8 July 2019.
- ↑ "Bourke Award 2019" . Retrieved 10 July 2019.
- ↑ "Murray Goodman Memorial Prize 2018". 5 November 2018.
- ↑ "Florida Award 2017".
- ↑ "Herty Medal 2015". Archived from the original on 2019-07-10. Retrieved 2019-07-10.
- ↑ "Feynman Prize 2013". Archived from the original on 2019-07-10. Retrieved 2019-07-10.
- ↑ "Hirschmann Visiting Professorship".
- ↑ "NSF Young Investigator: Electron Transfer in Complex Systems".