Related Research Articles

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.
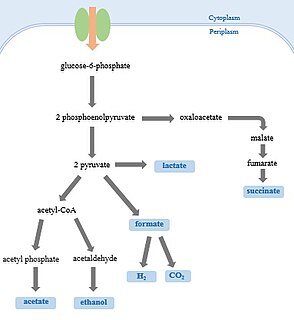
Mixed acid fermentation is the biological process by which a six-carbon sugar e.g. glucose is converted into a complex and variable mixture of acids. It is an anaerobic fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.

Spot 42 (spf) RNA is a regulatory non-coding bacterial small RNA encoded by the spf gene. Spf is found in gammaproteobacteria and the majority of experimental work on Spot42 has been performed in Escherichia coli and recently in Aliivibrio salmonicida. In the cell Spot42 plays essential roles as a regulator in carbohydrate metabolism and uptake, and its expression is activated by glucose, and inhibited by the cAMP-CRP complex.

In molecular biology, the protein domain SAICAR synthase is an enzyme which catalyses a reaction to create SAICAR. In enzymology, this enzyme is also known as phosphoribosylaminoimidazolesuccinocarboxamide synthase. It is an enzyme that catalyzes the chemical reaction

The E. coli long-term evolution experiment (LTEE) is an ongoing study in experimental evolution led by Richard Lenski that has been tracking genetic changes in 12 initially identical populations of asexual Escherichia coli bacteria since 24 February 1988. The populations reached the milestone of 50,000 generations in February 2010. Lenski performed the 10,000th transfer of the experiment on March 13, 2017. The populations reached 73,500 generations in early 2020, shortly before being frozen because of the COVID-19 pandemic. On September 2020, the LTEE experiment was resumed using the frozen stocks.
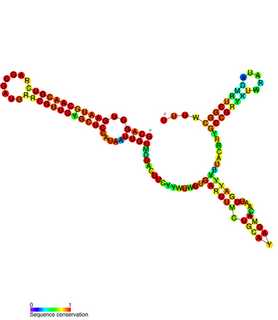
FnrS RNA is a family of Hfq-binding small RNA whose expression is upregulated in response to anaerobic conditions. It is named FnrS because its expression is strongly dependent on fumarate and nitrate reductase regulator (FNR), a direct oxygen availability sensor.
Tripartite ATP-independent periplasmic transporters are a large family of solute transporters found in bacteria and archaea, but not in eukaryotes, that appear to be specific for the uptake of organic acids or related molecules containing a carboxylate or sulfonate group. They are unique in that they utilize a substrate binding protein (SBP) in combination with a secondary transporter.
In molecular biology, the cytochrome c assembly protein family includes various proteins involved in cytochrome c assembly from mitochondria and bacteria. Members of this family include: CycK from Rhizobium leguminosarum, CcmC from Escherichia coli and Paracoccus denitrificans, and orf240 from Triticum aestivum (Wheat) mitochondria. The members of this family are probably integral membrane proteins with six predicted transmembrane helices that may comprise the membrane component of an ABC transporter complex. This transporter may be necessary for transport of some component needed for cytochrome c assembly. One member, R. leguminosarum CycK, contains a putative haem-binding motif. Wheat orf240 also contains a putative haem-binding motif and is a proposed ABC transporter with c-type haem as its proposed substrate. However it seems unlikely that all members of this family transport haem or c-type apocytochromes because P. denitrificans CcmC transports neither.
The Nucleobase cation symporter-2 (NCS2) family, also called the Nucleobase ascorbate transporter (NAT) family, consists of over 1000 sequenced proteins derived from gram-negative and gram-positive bacteria, archaea, fungi, plants and animals. The NCS2/NAT family is a member of the APC Superfamily of secondary carriers. Of the five known families of transporters that act on nucleobases, NCS2/NAT is the only one that is most widespread. Many functionally characterized members are specific for nucleobases including both purines and pyrimidines, but others are purine-specific. However, two closely related rat/human members of the family, SVCT1 and SVCT2, localized to different tissues of the body, co-transport L-ascorbate (vitamin C) and Na+ with a high degree of specificity and high affinity for the vitamin. Clustering of NCS2/NAT family members on the phylogenetic tree is complex, with bacterial proteins and eukaryotic proteins each falling into at least three distinct clusters. The plant and animal proteins cluster loosely together, but the fungal proteins branch from one of the three bacterial clusters forming a tighter grouping. E. coli possesses four distantly related paralogous members of the NCS2 family.
Cation diffusion facilitators (CDFs) are transmembrane proteins that provide tolerance of cells to divalent metal ions, such as cadmium, zinc, and cobalt. These proteins are considered to be efflux pumps that remove these divalent metal ions from cells. However, some members of the CDF superfamily are implicated in ion uptake. All members of the CDF family possess six putative transmembrane spanners with strongest conservation in the four N-terminal spanners. The Cation Diffusion Facilitator (CDF) Superfamily includes the following families:

Fumarate reductase (quinol) is an enzyme with systematic name succinate:quinone oxidoreductase. This enzyme catalyzes the following chemical reaction:
Proteins currently known to belong to the Ni2+-Co2+ Transporter (NiCoT) family (TC# 2.A.52) can be found in organisms ranging from Gram-negative and Gram-positive bacteria to archaea and some eukaryotes. Members of this family catalyze uptake of Ni2+ and/or Co2+ in a proton motive force-dependent process.
The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) flippase superfamily is a group of integral membrane protein families. The MOP flippase superfamily includes twelve distantly related families, six for which functional data are available:
- One ubiquitous family (MATE) specific for drugs - (TC# 2.A.66.1) The Multi Antimicrobial Extrusion (MATE) Family
- One (PST) specific for polysaccharides and/or their lipid-linked precursors in prokaryotes - (TC# 2.A.66.2) The Polysaccharide Transport (PST) Family
- One (OLF) specific for lipid-linked oligosaccharide precursors of glycoproteins in eukaryotes - (TC# 2.A.66.3) The Oligosaccharidyl-lipid Flippase (OLF) Family
- One (MVF) lipid-peptidoglycan precursor flippase involved in cell wall biosynthesis - (TC# 2.A.66.4) The Mouse Virulence Factor (MVF) Family
- One (AgnG) which includes a single functionally characterized member that extrudes the antibiotic, Agrocin 84 - (TC# 2.A.66.5) The Agrocin 84 Antibiotic Exporter (AgnG) Family
- And finally, one (Ank) that shuttles inorganic pyrophosphate (PPi) - (TC# 2.A.66.9) The Progressive Ankylosis (Ank) Family
The iron/lead transporter (ILT) family is a family of transmembrane proteins within the lysine exporter (LysE) superfamily. The ILT family includes two subfamilies, the iron-transporting (OFeT) family and the lead-transporting (PbrT) family. A representative list of the proteins belonging to these subfamilies of the ILT family can be found in the Transporter Classification Database.
The Nickel/Cobalt Transporter (NicO) Family is a member of the Lysine Exporter (LysE) Superfamily.
The Disulfide bond oxidoreductase D (DsbD) family is a member of the Lysine Exporter (LysE) Superfamily. A representative list of proteins belonging to the DsbD family can be found in the Transporter Classification Base.
The p-aminobenzoyl-glutamate transporter(AbgT) family is a family of transporter proteins belonging to the ion transporter (IT) superfamily. The AbgT family consists of the AbgT protein of E. coli and the MtrF drug exporter of Neisseria gonorrhoeae. The former protein is apparently cryptic in wild-type cells, but when expressed on a high copy number plasmid, or when expressed at higher levels due to mutation, it appeared to allow uptake and subsequent utilization of p-aminobenzoyl-glutamate as a source of p-aminobenzoate for p-aminobenzoate auxotrophs. p-Aminobenzoate is a constituent of and a precursor for the biosynthesis of folic acid. MtrF was annotated as a putative drug efflux pump.
The C4-dicarboxylate uptake C family or DcuC family (TC# 2.A.61) is a family of transmembrane ion transporters found in bacteria. A representative list of proteins belonging to the DcuC family can be found in the Transporter Classification Database.
The NhaB family belongs to the ion transporter (IT) superfamily. A representative list of proteins belonging to the NhaB family can be found in the Transporter Classification Database.
The Monovalent Cation:Proton Antiporter-2 (CPA2) Family is a moderately large family of transporters belonging to the CPA superfamily. Members of the CPA2 family have been found in bacteria, archaea and eukaryotes. The proteins of the CPA2 family consist of between 333 and 900 amino acyl residues and exhibit 10-14 transmembrane α-helical spanners (TMSs).
References
- ↑ Zientz E, Six S, Unden G (December 1996). "Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: roles of the three Dcu carriers in uptake and exchange". J. Bacteriol. 178 (24): 7241–7. doi:10.1128/jb.178.24.7241-7247.1996. PMC 178639 . PMID 8955408.
- ↑ Golby P, Kelly DJ, Guest JR, Andrews SC (September 1998). "Topological analysis of DcuA, an anaerobic C4-dicarboxylate transporter of Escherichia coli". J. Bacteriol. 180 (18): 4821–7. doi:10.1128/JB.180.18.4821-4827.1998. PMC 107505 . PMID 9733683.
- ↑ Six S, Andrews SC, Roberts RE, Unden G, Guest JR (November 1993). "Construction and properties of Escherichia coli mutants defective in two genes encoding homologous membrane proteins with putative roles in anaerobic C4-dicarboxylic acid transport". Biochem. Soc. Trans. 21 (4): 342S. doi:10.1042/bst021342s. PMID 8131924.
- ↑ Nogrady N, Imre A, Rychlik I, Barrow PA, Nagy B (December 2003). "Genes responsible for anaerobic fumarate and arginine metabolism are involved in growth suppression in Salmonella enterica serovar Typhimurium in vitro, without influencing colonisation inhibition in the chicken in vivo". Vet. Microbiol. 97 (3–4): 191–9. doi:10.1016/j.vetmic.2003.08.011. PMID 14654290.
- ↑ Ullmann R, Gross R, Simon J, Unden G, Kroger A (October 2000). "Transport of C(4)-dicarboxylates in Wolinella succinogenes". J. Bacteriol. 182 (20): 5757–64. doi:10.1128/jb.182.20.5757-5764.2000. PMC 94697 . PMID 11004174.
- ↑ Six S, Andrews SC, Unden G, Guest JR (November 1994). "Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct)". J. Bacteriol. 176 (21): 6470–8. doi:10.1128/jb.176.21.6470-6478.1994. PMC 197000 . PMID 7961398.
- ↑ Engel, P.; Krämer, R.; Unden, G. (1994-06-01). "Transport of C4-dicarboxylates by anaerobically grown Escherichia coli. Energetics and mechanism of exchange, uptake and efflux". European Journal of Biochemistry. 222 (2): 605–614. doi:10.1111/j.1432-1033.1994.tb18903.x. ISSN 0014-2956. PMID 8020497.
- 1 2 Golby P, Kelly DJ, Guest JR, Andrews SC (December 1998). "Transcriptional regulation and organization of the dcuA and dcuB genes, encoding homologous anaerobic C4-dicarboxylate transporters in Escherichia coli". J. Bacteriol. 180 (24): 6586–96. doi:10.1128/JB.180.24.6586-6596.1998. PMC 107762 . PMID 9852003.
- ↑ Engel P, Kramer R, Unden G (September 1992). "Anaerobic fumarate transport in Escherichia coli by an fnr-dependent dicarboxylate uptake system which is different from the aerobic dicarboxylate uptake system". J. Bacteriol. 174 (17): 5533–9. doi:10.1128/jb.174.17.5533-5539.1992. PMC 206496 . PMID 1512189.
- ↑ Golby P, Davies S, Kelly DJ, Guest JR, Andrews SC (February 1999). "Identification and characterization of a two-component sensor-kinase and response-regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli". J. Bacteriol. 181 (4): 1238–48. doi:10.1128/JB.181.4.1238-1248.1999. PMC 93502 . PMID 9973351.
- ↑ Zientz E, Bongaerts J, Unden G (October 1998). "Fumarate regulation of gene expression in Escherichia coli by the DcuSR (dcuSR genes) two-component regulatory system". J. Bacteriol. 180 (20): 5421–5. doi:10.1128/JB.180.20.5421-5425.1998. PMC 107591 . PMID 9765574.