Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

Oxytetracycline is a broad-spectrum tetracycline antibiotic, the second of the group to be discovered.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis activity. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified, and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin. The tuberactinomycins target bacterial ribosomes, binding RNA and disrupting bacterial protein synthesis and certain forms of RNA splicing. Viomycin is produced by the actinomycete Streptomyces puniceus.

'Novobiocin, also known as albamycin or ', is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

The avermectins are a series of drugs and pesticides used to treat parasitic worm infestations and to reduce insect pests. They are a group of 16-membered macrocyclic lactone derivatives with potent anthelmintic and insecticidal properties. These naturally occurring compounds are generated as fermentation products by Streptomyces avermitilis, a soil actinomycete. Eight different avermectins were isolated in four pairs of homologue compounds, with a major (a-component) and minor (b-component) component usually in ratios of 80:20 to 90:10. Avermectin B1, a mixture of B1a and B1b, is the drug and pesticide abamectin. Other anthelmintics derived from the avermectins include ivermectin, selamectin, doramectin, eprinomectin.

The PT-barrel, is a novel protein fold that was discovered in the crystal structure of the prenyltransferase, Orf2 from Streptomyces sp. strain CL190.
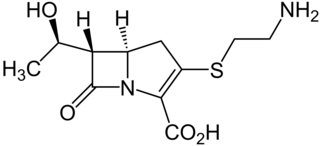
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.

Scytonemin is a secondary metabolite and an extracellular matrix (sheath) pigment synthesized by many strains of cyanobacteria, including Nostoc, Scytonema, Calothrix, Lyngbya, Rivularia, Chlorogloeopsis, and Hyella. Scytonemin-synthesizing cyanobacteria often inhabit highly insolated terrestrial, freshwater and coastal environments such as deserts, semideserts, rocks, cliffs, marine intertidal flats, and hot springs.

Geosmin synthase or germacradienol-geosmin synthase designates a class of bifunctional enzymes that catalyze the conversion of farnesyl diphosphate (FPP) to geosmin, a volatile organic compound known for its earthy smell. The N-terminal half of the protein catalyzes the conversion of farnesyl diphosphate to germacradienol and germacrene D, followed by the C-terminal-mediated conversion of germacradienol to geosmin. The conversion of FPP to geosmin was previously thought to involve multiple enzymes in a biosynthetic pathway.

Marinone is an antibiotic made by marine actinomycetes.

Salinispora is a genus of obligately aerobic, gram-positive, non-acid-fast bacteria belonging to the family of Micromonosporaceae. They are heterotrophic, non-motile, and obligately grow under high osmotic/ionic-strength conditions. They are the first identified genus of gram-positive bacteria which has a high osmotic/ionic-strength requirement for survival. They are widely abundant in tropical marine sediments and were first identified in 2002. This genus of bacteria has potential biotechnological significance due to their production of novel secondary metabolites which can be used pharmaceutically.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
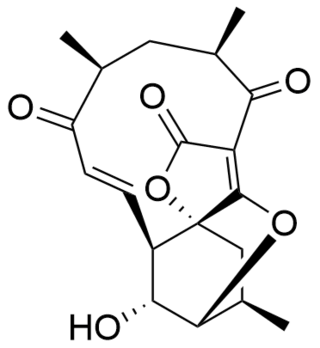
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB, thereby depleting the biosynthesis of p-aminobenzoate.
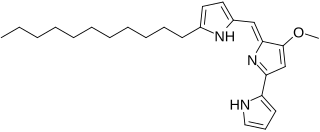
Undecylprodigiosin is an alkaloid produced by some Actinomycetes bacteria. It is a member of the prodiginines group of natural products and has been investigated for potential antimalarial activity.

Enterocin and its derivatives are bacteriocins synthesized by the lactic acid bacteria, Enterococcus. This class of polyketide antibiotics are effective against foodborne pathogens including L. monocytogenes, Listeria, and Bacillus. Due to its proteolytic degradability in the gastrointestinal tract, enterocin is used for controlling foodborne pathogens via human consumption.

















