
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.

Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, which cannot be made in the body. It is found in food and commercially synthesized to be a dietary supplement or medication. Food sources of thiamine include whole grains, legumes, and some meats and fish. Grain processing removes much of the thiamine content, so in many countries cereals and flours are enriched with thiamine. Supplements and medications are available to treat and prevent thiamine deficiency and disorders that result from it, including beriberi and Wernicke encephalopathy. Other uses include the treatment of maple syrup urine disease and Leigh syndrome. They are typically taken by mouth, but may also be given by intravenous or intramuscular injection.
Serine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.

In molecular biology, a riboswitch is a regulatory segment of a messenger RNA molecule that binds a small molecule, resulting in a change in production of the proteins encoded by the mRNA. Thus, an mRNA that contains a riboswitch is directly involved in regulating its own activity, in response to the concentrations of its effector molecule. The discovery that modern organisms use RNA to bind small molecules, and discriminate against closely related analogs, expanded the known natural capabilities of RNA beyond its ability to code for proteins, catalyze reactions, or to bind other RNA or protein macromolecules.

Anisomycin, also known as flagecidin, is an antibiotic produced by Streptomyces griseolus which inhibits eukaryotic protein synthesis. Partial inhibition of DNA synthesis occurs at anisomycin concentrations that effect 95% inhibition of protein synthesis. Anisomycin can activate stress-activated protein kinases, MAP kinase and other signal transduction pathways.
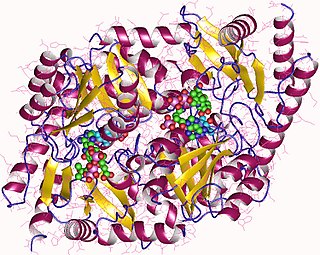
Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme (EC 2.3.1.37) that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes, cobalamins and chlorophylls. The reaction is as follows:
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can only synthesize 11 of the 20 standard amino acids, and in time of accelerated growth, histidine can be considered an essential amino acid.

L-Arginine:glycine amidinotransferase is the enzyme that catalyses the transfer of an amidino group from L-arginine to glycine. The products are L-ornithine and glycocyamine, also known as guanidinoacetate, the immediate precursor of creatine. Creatine and its phosphorylated form play a central role in the energy metabolism of muscle and nerve tissues. Creatine is in highest concentrations in the skeletal muscle, heart, spermatozoa and photoreceptor cells. Creatine helps buffer the rapid changes in ADP/ATP ratio in muscle and nerve cells during active periods. Creatine is also synthesized in other tissues, such as pancreas, kidneys, and liver, where amidinotransferase is located in the cytoplasm, including the intermembrane space of the mitochondria, of the cells that make up those tissues.

Phosphoribosylamine (PRA) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from PRA.
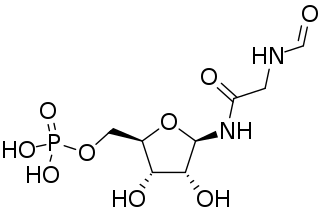
Phosphoribosyl-N-formylglycineamide is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from FGAR.
In enzymology, a glycine dehydrogenase (cyanide-forming) (EC 1.4.99.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a 1-deoxy-d-xylulose-5-phosphate synthase (EC 2.2.1.7) is an enzyme in the non-mevalonate pathway that catalyzes the chemical reaction

5′-Phosphoribosyl-5-aminoimidazole is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from AIR. It is an intermediate in the adenine pathway and is synthesized from 5′-phosphoribosylformylglycinamidine by AIR synthetase.
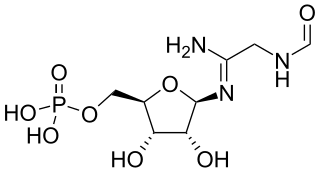
5′-Phosphoribosylformylglycinamidine is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from FGAM.

Glycineamide ribonucleotide is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from GAR.
Sulfur carrier protein ThiS adenylyltransferase is an enzyme with systematic name ATP:(ThiS) adenylyltransferase. This enzyme catalyses the following chemical reaction

Thiazole synthase (EC 2.8.1.10, thiG (gene)) is an enzyme with systematic name 1-deoxy-D-xylulose 5-phosphate:thiol sulfurtransferase. This enzyme catalyses the following chemical reaction
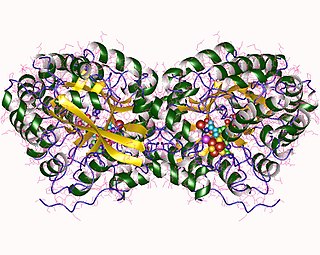
Phosphomethylpyrimidine synthase is an enzyme with systematic name 5-amino-1-(5-phospho-D-ribosyl)imidazole formate-lyase . This enzyme catalyses the following chemical reaction

Within the field of biochemistry, 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP) also known as toxopyrimidine together with its mono phosphate (HMP-P) and pyrophosphate (HMP-PP) esters are biogenetic precursors to the important biochemical cofactor thiamine pyrophosphate (TPP), a derivative of thiamine (vitamin B1).















