Related Research Articles

An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.

Ammonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world's food and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.

An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
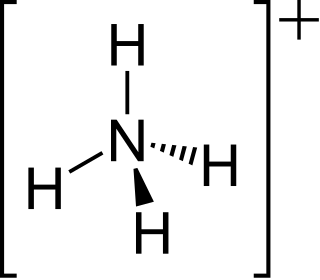
The ammonium cation is a positively charged polyatomic ion with the chemical formula [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic groups.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances which react with acids as originally proposed by G.-F. Rouelle in the mid-18th century.

Tanning is the process of treating skins and hides of animals to produce leather. A tannery is the place where the skins are processed.

Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.

Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia.

In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.

Alkalinity is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
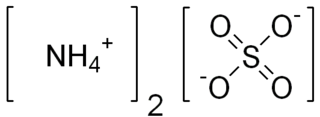
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A half-neutralization occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions to create water molecules.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Although no evidence has been presented for the existence of this species in solution or as a solid, aqueous solutions of hexafluorosilicic acid are well-defined, consisting of salts derived from various protonated forms of water as the cation and hexafluorosilicate as the anion. These salts and their aqueous solutions are colorless.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.
The Kjeldahl method or Kjeldahl digestion (Danish pronunciation: [ˈkʰelˌtɛˀl]) in analytical chemistry is a method for the quantitative determination of nitrogen contained in organic substances plus the nitrogen contained in the inorganic compounds ammonia and ammonium (NH3/NH4+). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen content and protein content it is an important method for analyzing proteins. This method was developed by Johan Kjeldahl in 1883.
Liming is a process used for parchment or leather processing, in which hides are soaked in an alkali solution. It is performed using a drum and paddle or a pit. Its objectives are:

Alkali, or Alkaline, soils are clay soils with high pH, a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swell and difficult to clarify/settle. They derive their name from the alkali metal group of elements, to which sodium belongs, and which can induce basicity. Sometimes these soils are also referred to as alkaline sodic soils.
Alkaline soils are basic, but not all basic soils are alkaline.
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process; it produces stronger fibers and is less environmentally costly.
References
- ↑ Sharphouse, J.H. (1983), Leather Technician's Handbook, Leather Producer's Association, p. 104, ISBN 0-9502285-1-6
