
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Deuterium is one of two stable isotopes of hydrogen. The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom in 6420 of hydrogen. Thus deuterium accounts for approximately 0.0156% of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another.

The giant planets constitute a diverse type of planet much larger than Earth. They are usually primarily composed of low-boiling-point materials (volatiles), rather than rock or other solid matter, but massive solid planets can also exist. There are four known giant planets in the Solar System: Jupiter, Saturn, Uranus and Neptune. Many extrasolar giant planets have been identified orbiting other stars.

Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H2. It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen each atom has one proton, one electron, and no neutrons.

The halogens are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The artificially created element 117, tennessine (Ts), may also be a halogen. In the modern IUPAC nomenclature, this group is known as group 17.

Heavy water is a form of water that contains only deuterium rather than the common hydrogen-1 isotope that makes up most of the hydrogen in normal water. The presence of the heavier hydrogen isotope gives the water different nuclear properties, and the increase in mass gives it slightly different physical and chemical properties when compared to normal water.

Tritium or hydrogen-3 is a rare and radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (protium) contains one proton and zero neutrons, and that of hydrogen-2 (deuterium) contains one proton and one neutron.
Light water or Lightwater may refer to:

Harold Clayton Urey was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934 for the discovery of deuterium. He played a significant role in the development of the atom bomb, as well as contributing to theories on the development of organic life from non-living matter.

Semiheavy water is the result of replacing one of the protium in light water with deuterium. It exists whenever there is water with light hydrogen (protium, 1H) and deuterium (D or 2H) in the mix. This is because hydrogen atoms (hydrogen-1 and deuterium) are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen contains about 50% HDO and 25% each of H2O and D2O, in dynamic equilibrium. In regular water, about 1 molecule in 3,200 is HDO (one hydrogen in 6,400 is D). By comparison, heavy water D2O occurs at a proportion of about 1 molecule in 41 million (i.e., one in 6,4002). This makes semiheavy water far more common than "normal" heavy water.

The Girdler sulfide (GS) process, also known as the Geib–Spevack (GS) process, is an industrial production method for filtering out of natural water the heavy water (deuterium oxide = D2O) which is used in particle research, in deuterium NMR spectroscopy, deuterated solvents for proton NMR spectroscopy, in heavy water nuclear reactors (as a coolant and moderator) and in deuterated drugs.

Hydrogen (1H) has three naturally occurring isotopes, sometimes denoted 1
H
, 2
H
, and 3
H
. 1
H
and 2
H
are stable, while 3
H
has a half-life of 12.32(2) years. Heavier isotopes also exist, all of which are synthetic and have a half-life of less than one zeptosecond (10−21 s). Of these, 5
H
is the least stable, while 7
H
is the most.
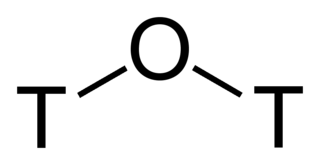
Tritiated water is a radioactive form of water in which the usual protium atoms are replaced with tritium. In its pure form it may be called tritium oxide (T2O or 3H2O) or super-heavy water. Pure T2O is corrosive due to self-radiolysis. Diluted, tritiated water is mainly H2O plus some HTO (3HOH). It is also used as a tracer for water transport studies in life-science research. Furthermore, since it naturally occurs in minute quantities, it can be used to determine the age of various water-based liquids, such as vintage wines.

The origin of water on Earth is the subject of a body of research in the fields of planetary science, astronomy, and astrobiology. Earth is unique among the rocky planets in the Solar System in that it is the only planet known to have oceans of liquid water on its surface. Liquid water, which is necessary for life as we know it, continues to exist on the surface of Earth because the planet is at a distance, known as the habitable zone, far enough from the Sun that it does not lose its water, but not so far that low temperatures cause all water on the planet to freeze.
Doubly labeled water is water in which both the hydrogen and the oxygen have been partly or completely replaced with an uncommon isotope of these elements for tracing purposes.

Water is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. It is also the third most abundant molecule in the universe.
Deuterium-depleted water (DDW) is water which has a lower concentration of deuterium than occurs naturally at sea level on Earth.
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water (deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The heavy water coolant is kept under pressure to avoid boiling, allowing it to reach higher temperature (mostly) without forming steam bubbles, exactly as for pressurized water reactor. While heavy water is very expensive to isolate from ordinary water (often referred to as light water in contrast to heavy water), its low absorption of neutrons greatly increases the neutron economy of the reactor, avoiding the need for enriched fuel. The high cost of the heavy water is offset by the lowered cost of using natural uranium and/or alternative fuel cycles. As of the beginning of 2001, 31 PHWRs were in operation, having a total capacity of 16.5 GW(e), representing roughly 7.76% by number and 4.7% by generating capacity of all current operating reactors.

The P-9 Project was the codename given during World War II to the Manhattan Project's heavy water production program. The Cominco operation at Trail, British Columbia, was upgraded to produce heavy water. DuPont built three plants in the United States: at the Morgantown Ordnance Works, near Morgantown, West Virginia; at the Wabash River Ordnance Works, near Dana and Newport, Indiana; and at the Alabama Ordnance Works, near Childersburg and Sylacauga, Alabama. The American plants operated from 1943 until 1945. The Canadian plant at Trail continued in operation until 1956. Three nuclear reactors were built using the heavy water produced by the P-9 Project: Chicago Pile 3 at Argonne, and ZEEP and NRX at the Chalk River Laboratories in Canada.
Hydrogen chalcogenides are binary compounds of hydrogen with chalcogen atoms. Water, the first chemical compound in this series, contains one oxygen atom and two hydrogen atoms, and is the most common compound on the Earth's surface.















