Related Research Articles
An allele is a variant of the sequence of nucleotides at a particular location, or locus, on a DNA molecule.

In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and the second is called recessive. This state of having two different variants of the same gene on each chromosome is originally caused by a mutation in one of the genes, either new or inherited. The terms autosomal dominant or autosomal recessive are used to describe gene variants on non-sex chromosomes (autosomes) and their associated traits, while those on sex chromosomes (allosomes) are termed X-linked dominant, X-linked recessive or Y-linked; these have an inheritance and presentation pattern that depends on the sex of both the parent and the child. Since there is only one copy of the Y chromosome, Y-linked traits cannot be dominant or recessive. Additionally, there are other forms of dominance, such as incomplete dominance, in which a gene variant has a partial effect compared to when it is present on both chromosomes, and co-dominance, in which different variants on each chromosome both show their associated traits.
Suxamethonium chloride, also known as suxamethonium or succinylcholine, or simply sux in medical abbreviation, is a medication used to cause short-term paralysis as part of general anesthesia. This is done to help with tracheal intubation or electroconvulsive therapy. It is administered by injection, either into a vein or into a muscle. When used in a vein, onset of action is generally within one minute and effects last for up to 10 minutes.
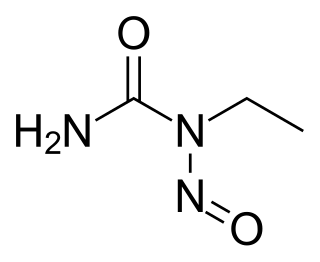
ENU, also known as N-ethyl-N-nitrosourea (chemical formula C3H7N3O2), is a highly potent mutagen. For a given gene in mice, ENU can induce 1 new mutation in every 700 loci. It is also toxic at high doses.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
A heterozygote advantage describes the case in which the heterozygous genotype has a higher relative fitness than either the homozygous dominant or homozygous recessive genotype. Loci exhibiting heterozygote advantage are a small minority of loci. The specific case of heterozygote advantage due to a single locus is known as overdominance. Overdominance is a rare condition in genetics where the phenotype of the heterozygote lies outside of the phenotypical range of both homozygote parents, and heterozygous individuals have a higher fitness than homozygous individuals.

Overdominance is a phenomenon in genetics where the phenotype of the heterozygote lies outside the phenotypical range of both homozygous parents. Overdominance can also be described as heterozygote advantage regulated by a single genomic locus, wherein heterozygous individuals have a higher fitness than homozygous individuals. However, not all cases of the heterozygote advantage are considered overdominance, as they may be regulated by multiple genomic regions. Overdominance has been hypothesized as an underlying cause for heterosis.
Pseudocholinesterase deficiency is an autosomal recessive inherited blood plasma enzyme abnormality in which the body's production of butyrylcholinesterase is impaired. People who have this abnormality may be sensitive to certain anesthetic drugs, including the muscle relaxants succinylcholine and mivacurium as well as other ester local anesthetics.

Genetic variation in populations can be analyzed and quantified by the frequency of alleles. Two fundamental calculations are central to population genetics: allele frequencies and genotype frequencies. Genotype frequency in a population is the number of individuals with a given genotype divided by the total number of individuals in the population. In population genetics, the genotype frequency is the frequency or proportion of genotypes in a population.
Genetics, a discipline of biology, is the science of heredity and variation in living organisms.
Mutation–selection balance is an equilibrium in the number of deleterious alleles in a population that occurs when the rate at which deleterious alleles are created by mutation equals the rate at which deleterious alleles are eliminated by selection. The majority of genetic mutations are neutral or deleterious; beneficial mutations are relatively rare. The resulting influx of deleterious mutations into a population over time is counteracted by negative selection, which acts to purge deleterious mutations. Setting aside other factors, the equilibrium number of deleterious alleles is then determined by a balance between the deleterious mutation rate and the rate at which selection purges those mutations.
A null allele is a nonfunctional allele caused by a genetic mutation. Such mutations can cause a complete lack of production of the associated gene product or a product that does not function properly; in either case, the allele may be considered nonfunctional. A null allele cannot be distinguished from deletion of the entire locus solely from phenotypic observation.

Triosephosphate isomerase deficiency is a rare autosomal recessive metabolic disorder which was initially described in 1965.
In medical genetics, compound heterozygosity is the condition of having two or more heterogeneous recessive alleles at a particular locus that can cause genetic disease in a heterozygous state; that is, an organism is a compound heterozygote when it has two recessive alleles for the same gene, but with those two alleles being different from each other. Compound heterozygosity reflects the diversity of the mutation base for many autosomal recessive genetic disorders; mutations in most disease-causing genes have arisen many times. This means that many cases of disease arise in individuals who have two unrelated alleles, who technically are heterozygotes, but both the alleles are defective.
Lethal alleles are alleles that cause the death of the organism that carries them. They are usually a result of mutations in genes that are essential for growth or development. Lethal alleles can be recessive, dominant, conditional, perinatal, or postnatal after an extended period of apparently normal development depending on the gene or genes involved.
Butyrylcholinesterase, also known asBChE, BuChE, BuChase, pseudocholinesterase, or plasma (cholin)esterase, is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma, and encoded by the BCHE gene.
The infinite alleles model is a mathematical model for calculating genetic mutations. The Japanese geneticist Motoo Kimura and American geneticist James F. Crow (1964) introduced the infinite alleles model, an attempt to determine for a finite diploid population what proportion of loci would be homozygous. This was, in part, motivated by assertions by other geneticists that more than 50 percent of Drosophila loci were heterozygous, a claim they initially doubted. In order to answer this question they assumed first, that there were a large enough number of alleles so that any mutation would lead to a different allele ; and second, that the mutations would result in a number of different outcomes from neutral to deleterious.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

Zygosity is the degree to which both copies of a chromosome or gene have the same genetic sequence. In other words, it is the degree of similarity of the alleles in an organism.

In poultry standards, solid white is coloration of plumage in chickens characterized by a uniform pure white color across all feathers, which is not generally associated with depigmentation in any other part of the body.
References
- ↑ Kalow, W; Genest, K (1957). "A method for the detection of atypical forms of human serum cholinesterase: Determination of dibucaine". Can. J. Biochem. 35 (s): 339–46. doi:10.1139/o57-041.
- 1 2 3 Miller, R (2005). Miller's Anesthesia (6th ed.). Philadelphia: Elsevier.
- ↑ McGuire, MC; Nogueira, CP; Bartels, CF; Lightstone, H; Hajra, A; Van der Spek, AF; Lockridge, O; La Du, BN (February 1989). "Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase". Proc Natl Acad Sci USA. 86 (3): 953–957. Bibcode:1989PNAS...86..953M. doi: 10.1073/pnas.86.3.953 . PMC 286597 . PMID 2915989.
- ↑ Gaffney, D; Campbell, RA (March 1994). "A PCR based method to determine the Kalow allele of the cholinesterase gene: the E1k allele frequency and its significance in the normal population". J Med Genet. 31 (3): 248–250. doi:10.1136/jmg.31.3.248. PMC 1049753 . PMID 8014977.
- ↑ Lockridge, O; La Du, BN (25 January 1978). "Comparison of atypical and usual human serum cholinesterase. Purification, number of active sites, substrate affinity, and turnover number". J Biol Chem. 253 (2): 361–6. doi: 10.1016/S0021-9258(17)38214-5 . PMID 618874.
- ↑ Pestel, G; Sprenger, H; Rothhammer, A (June 2003). "Frequency distribution of dibucaine numbers in 24,830 patients". Der Anaesthesist. 52 (6): 495–9. doi:10.1007/s00101-003-0497-8. PMID 12835869.