
Alder is the common name of a genus of flowering plants (Alnus) belonging to the birch family Betulaceae. The genus comprises about 35 species of monoecious trees and shrubs, a few reaching a large size, distributed throughout the north temperate zone with a few species extending into Central America, as well as the northern and southern Andes.
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. The reaction is known for its mild character and wide tolerance of functional groups.
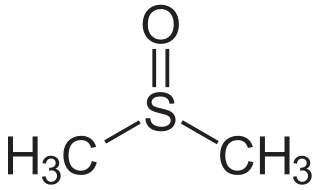
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high melting point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after contact with the skin.
Dimethyl ether (DME, also known as methoxymethane) is the organic compound with the formula CH3OCH3, simplified to C2H6O. The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. It is an isomer of ethanol.

Dimethyl sulfate is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or even Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at 37 °C (99 °F) and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol.

N,N-Dimethyllysergamide or N,N-dimethyl-D-lysergamide (DAM-57) is a derivative of ergine. There has been a single report of observing N,N-dimethyl-D-lysergamide in the illicit drug market. This compound did induce autonomic disturbances at oral levels of some ten times the dosage required for LSD, presumably in the high hundreds of micrograms. There is some disagreement as to whether there were psychic changes observed.

Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed as a dienophile in cycloaddition reactions, such as the Diels-Alder reaction. It is also a potent Michael acceptor. This compound exists as a colorless liquid at room temperature. This compound was used in the preparation of nedocromil.

Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (FSO2OCH3).

Dimethyl fumarate (DMF) is the methyl ester of fumaric acid and is named after the earth smoke plant. DMF combined with three other fumaric acid esters (FAEs) is solely licensed in Germany as an oral therapy for psoriasis. Since 2013, it has been used to treat adults with relapsing multiple sclerosis. In 2017, a new oral formulation of DMF was approved by the European Medicines Agency (EMA) for use in Europe as a treatment for moderate-to-severe plaque psoriasis. DMF is thought to have immunomodulatory properties without causing significant immunosuppression.
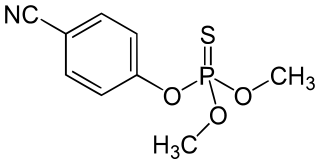
Cyanophos is a cholinesterase inhibitor used as an insecticide and avicide; for example, against rice stem borers and house flies. It is part of the chemical class of organophosphorus compounds, and is a yellow to reddish-yellow transparent liquid.
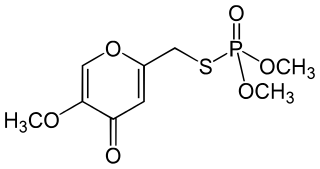
Endothion is an organic compound used as an insecticide and acaricides. It is part of the chemical class of organophosphorus compounds. It is generally described as white crystals with a slight odor. It is used as an insecticide, but not sold in the United States or Canada.

Dimethyl 4-(methylthio)phenyl phosphate is a chemical compound used as an insecticide and an acaricide.
Dimethylbutadiene, formally referred to as 2,3-dimethyl-1,3-butadiene, is an organic compound with the formula (CH3)2C4H4. It is colorless liquid which served an important role in the early history of synthetic rubber. It is now a specialty reagent.

Tetrachlorvinphos is an organophosphate insecticide used to kill fleas and ticks.
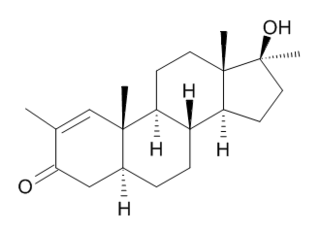
Methylstenbolone, known by the nicknames M-Sten, Methyl-Sten, and Ultradrol, is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-methylated derivative of dihydrotestosterone (DHT) which was never introduced for medical use. It is a designer steroid and has been sold "over-the-counter" via the internet as a "dietary/nutritional supplement".

Dimethandrolone buciclate, or dimethandrolone bucyclate, also known as 7α,11β-dimethyl-19-nortestosterone 17β-buciclate, is a synthetic anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was developed by the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development (NICHD) and has not been marketed at this time. It is an androgen ester – specifically, the C17β buciclate (4-butylcyclohexane-1-carboxylate) ester of dimethandrolone (7α,11β-dimethyl-19-nortestosterone) – and acts as a prodrug of dimethandrolone in the body. Dimethandrolone buciclate is or was under investigation as a potential male contraceptive.

Dimethandrolone dodecylcarbonate, or dimethandrolone dodecanoylcarbonate, also known as 7α,11β-dimethyl-19-nortestosterone 17β-dodecylcarbonate, is a synthetic and orally active anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was developed by the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development (NICHD) and has not been marketed at this time. It is an androgen ester – specifically, the C17β dodecylcarbonate ester of dimethandrolone (7α,11β-dimethyl-19-nortestosterone) – and acts as a prodrug of dimethandrolone in the body.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.

















