Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

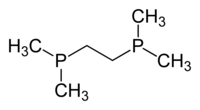
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.
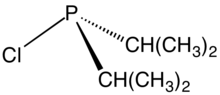
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).
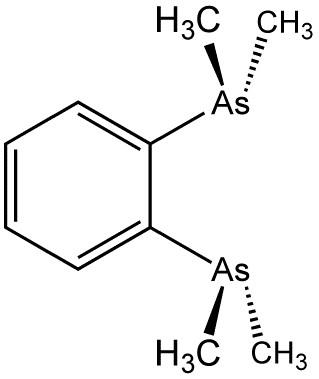
1,2-Bis(dimethylarsino)benzene (diars) is the organoarsenic compound with the formula C6H4(As(CH3)2)2. The molecule consists of two dimethylarsino groups attached to adjacent carbon centers of a benzene ring. It is a chelating ligand in coordination chemistry. This colourless oil is commonly abbreviated "diars."

In coordination chemistry, the bite angle is the angle on a central atom between two bonds to a bidentate ligand. This ligand–metal–ligand geometric parameter is used to classify chelating ligands, including those in organometallic complexes. It is most often discussed in terms of catalysis, as changes in bite angle can affect not just the activity and selectivity of a catalytic reaction but even allow alternative reaction pathways to become accessible.

Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.

1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.

Trans-spanning ligands are bidentate ligands that can span opposite sites of a complex with square-planar geometry. A wide variety of ligands that chelate in the cis fashion already exist, but very few can link opposite vertices on a coordination polyhedron. Early attempts to generate trans-spanning bidentate ligands relied on long hydrocarbon chains to link the donor functionalities, but such ligands often lead to coordination polymers.
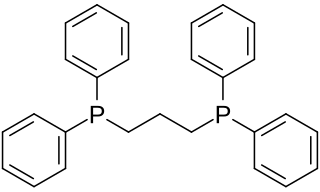
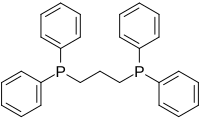
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands occupy coordination sites. Spectator ligands tend to be of polydentate, such that the M-spectator ensemble is inert kinetically. Although they do not participate in reactions of the metal, spectator ligands influence the reactivity of the metal center to which they are bound. These ligands are sometimes referred to as ancillary ligands.

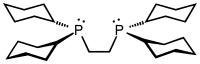
Bis(dicyclohexylphosphino)ethane, abbreviated dcpe, is an organophosphorus compound with the formula (C6H11)2PCH2CH2P(C6H11)2. It is a white solid that is soluble in nonpolar organic solvents. The compound is used as a bulky and highly basic diphosphine ligand in coordination chemistry.

1,5-Diaza-3,7-diphosphacyclooctanes are organophosphorus compounds with the formula [R'NCH2P(R)CH2]2, often abbreviated PR2NR'2. They are air-sensitive white solids that are soluble in organic solvents. The ligands exist as meso and d,l-diastereomers, but only the meso forms function as bidentate ligands.
In organophosphorus chemistry, aminophosphines are compounds with the formula R3−nP(NR2)n where R is a hydrogen or organic substituent, and n = 0, 1, or 2. At one extreme, the parents H2PNH2 and P(NH2)3 are lightly studied and fragile. At the other extreme, tris(dimethylamino)phosphine (P(NMe2)3) is commonly available. Intermediate members are known, such as Ph2PN(H)Ph. Aminophosphines are typically colorless and reactive to oxygen. Aminophosphines are pyramidal geometry at phosphorus.
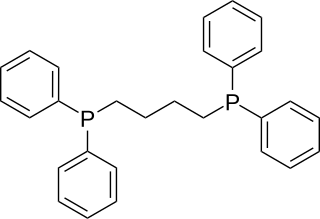
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.
1,2-Bis(dichlorophosphino)ethane is an organophosphorus compound with the formula (CH2PCl2)2. This colorless liquid is a precursor to chelating diphosphines.
1,2-Bis(diphenylphosphino)benzene (dppbz) is an organophosphorus compound with the formula C6H4(PPh2)2 (Ph = C6H5). Classified as a diphosphine ligand, it is a common bidentate ligand in coordination chemistry. It is a white, air-stable solid. As a chelating ligand, dppbz is very similar to 1,2-bis(diphenylphosphino)ethylene.
cis-1,2-Bis(diphenylphosphino)ethylene (dppv) is an organophosphorus compound with the formula C2H2(PPh2)2 (Ph = C6H5). Both the cis and trans isomers are known, but the cis isomer is of primary interest. Classified as a diphosphine ligand, it is a bidentate ligand in coordination chemistry. For example it gives rise to the complex Ni(dppv)2 and the coordination polymer [Ni(dppv)]n. As a chelating ligand, dppv is very similar to 1,2-bis(diphenylphosphino)benzene.