BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1′ positions. This C2-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation (atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle between the naphthyl groups is approximately 90°. The natural bite angle is 93°.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as: a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts.
Hydrocyanation is, most fundamentally, the process whereby H+ and –CN ions are added to a molecular substrate. Usually the substrate is an alkene and the product is a nitrile. When –CN is a ligand in a transition metal complex, its basicity makes it difficult to dislodge, so, in this respect, hydrocyanation is remarkable. Since cyanide is both a good σ–donor and π–acceptor its presence accelerates the rate of substitution of ligands trans from itself, the trans effect.1 A key step in hydrocyanation is the oxidative addition of hydrogen cyanide to low–valent metal complexes. In hydrocyanation of unsaturated carbonyls addition over the alkene competes with addition over the carbonyl group.
In chemistry, the Noyori asymmetric hydrogenation of ketones is a chemical reaction for the enantioselective hydrogenation of ketones, aldehydes, and imines. This reaction exploits using chiral ruthenium catalysts introduced by Ryoji Noyori. He shared half of the Nobel Prize in Chemistry in 2001 with William S. Knowles for the study of the asymmetric hydrogenation.

In organic chemistry, the term organocatalysis refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
In chemistry, bis(oxazoline) ligands (often abbreviated BOX ligands) are a class of privileged chiral ligands containing two oxazoline rings. They are typically C2‑symmetric and exist in a wide variety of forms; with structures based around CH2 or pyridine linkers being particularly common (often generalised BOX and PyBOX respectively). The coordination complexes of bis(oxazoline) ligands are used in asymmetric catalysis. These ligands are examples of C2-symmetric ligands.

DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen preferentially to one of two faces of an unsaturated substrate molecule, such as an alkene or ketone. The selectivity derives from the manner that the substrate binds to the chiral catalysts. In jargon, this binding transmits spatial information from the catalyst to the target, favoring the product as a single enantiomer. This enzyme-like selectivity is particularly applied to bioactive products such as pharmaceutical agents and agrochemicals.
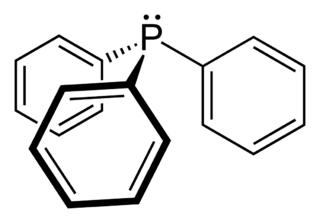
Phosphine ligands are phosphines, compound of the formula PRR'R" that are used as ligands in metal complexes, often related to organometallic chemistry and homogeneous catalysis. These compounds are also used in other areas of chemistry.

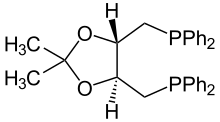
DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry. The first organogold compound discovered was gold(I) carbide Au2C2, which was first prepared in 1900.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective applications.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.
In chemistry, metal-catalysed hydroboration is a reaction used in organic synthesis. It is one of several examples of homogeneous catalysis.
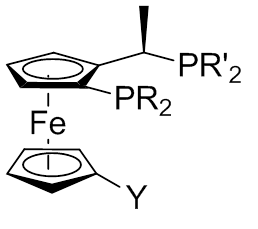
A Josiphos ligand is a type of chiral diphosphine which has been modified to be substrate-specific; they are widely used for enantioselective synthesis. They are named after the technician who made the first one, Josi Puleo.
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.

Trisoxazolines are a class of tridentate, chiral ligands composed of three oxazoline rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths, due to the chelate effect. The ligands have been investigated for molecular recognition and their complexes are used in asymmetric catalysts and polymerisation.

Ugi’s amine is a chemical compound named for the chemist who first reported its synthesis in 1970, Ivar Ugi. It is a ferrocene derivative. Since its first report, Ugi’s amine has found extensive use as the synthetic precursor to a large number of metal ligands that bear planar chirality. These ligands have since found extensive use in a variety of catalytic reactions. The compound may exist in either the 1S or 1R isomer, both of which have synthetic utility and are commercially available. Most notably, it is the synthetic precursor to the Josiphos class of ligands.
In homogeneous catalysis, a C2-symmetric ligands usually describes bidentate ligands that are dissymmetric but not asymmetric by virtue of their C2-symmetry. Such ligands have proven valuable in catalysis. With C2 symmetry, C2-symmetric ligands limit the number of possible reaction pathways and thereby increase enantioselectivity, at least relative to asymmetrical analogues. Chiral ligands combine with metals to form chiral catalyst, which engages in a chemical reaction in which chirality is transfer to the reaction product. C2 symmetric ligands are a subset of chiral ligands.

P-Chiral phosphines are organophosphorus compounds of the formula PRR′R″, where R, R′, R″ = H, alkyl, aryl, etc. They are a subset of chiral phosphines, a broader class of compounds where the stereogenic center can reside at sites other than phosphorus. P-chirality exploits the high barrier for inversion of phosphines, which ensures that enantiomers of PRR'R" do not racemize readily. The inversion barrier is relatively insensitive to substituents for triorganophosphines. By contrast, most amines of the type NRR′R″ undergo rapid pyramidal inversion.