In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.
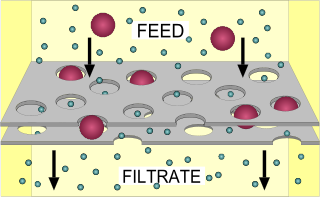
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.
Pervaporation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel. It may also refer to a device or system designed to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.
A bioprocess is a specific process that uses complete living cells or their components to obtain desired products.
Fast protein liquid chromatography (FPLC), is a form of liquid chromatography that is often used to analyze or purify mixtures of proteins. As in other forms of chromatography, separation is possible because the different components of a mixture have different affinities for two materials, a moving fluid and a porous solid. In FPLC the mobile phase is an aqueous solution, or "buffer". The buffer flow rate is controlled by a positive-displacement pump and is normally kept constant, while the composition of the buffer can be varied by drawing fluids in different proportions from two or more external reservoirs. The stationary phase is a resin composed of beads, usually of cross-linked agarose, packed into a cylindrical glass or plastic column. FPLC resins are available in a wide range of bead sizes and surface ligands depending on the application.

Protein A is a 49 kDa surface protein originally found in the cell wall of the bacteria Staphylococcus aureus. It is encoded by the spa gene and its regulation is controlled by DNA topology, cellular osmolarity, and a two-component system called ArlS-ArlR. It has found use in biochemical research because of its ability to bind immunoglobulins. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs. It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of the human VH3 family. Through these interactions in serum, where IgG molecules are bound in the wrong orientation, the bacteria disrupts opsonization and phagocytosis.
Expanded bed adsorption (EBA) is a preparative chromatographic technique which makes processing of viscous and particulate liquids possible.
Chromatography is a physical method of separation that distributes the components you want to separate between two phases, one stationary, the other moving in a definite direction. Cold ethanol precipitation, developed by Cohn in 1946, manipulates pH, ionic strength, ethanol concentration and temperature to precipitate different protein fractions from plasma. Chromatographic techniques utilise ion exchange, gel filtration and affinity resins to separate proteins. Since the 1980s it has emerged as an effective method of purifying blood components for therapeutic use.
Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) is a form of chromatography that is used to separate or purify biomolecules from complex mixtures. It was developed at the Swiss Federal Institute of Technology Zürich by Aumann and Morbidelli. The process consists of two to six chromatographic columns which are connected to one another in such a way that as the mixture moves through the columns the compound is purified into several fractions.

Acetone–butanol–ethanol (ABE) fermentation, also known as the Weizmann process, is a process that uses bacterial fermentation to produce acetone, n-butanol, and ethanol from carbohydrates such as starch and glucose. It was developed by chemist Chaim Weizmann and was the primary process used to produce acetone, which was needed to make cordite, a substance essential for the British war industry during World War I.
Displacement chromatography is a chromatography technique in which a sample is placed onto the head of the column and is then displaced by a solute that is more strongly sorbed than the components of the original mixture. The result is that the components are resolved into consecutive “rectangular” zones of highly concentrated pure substances rather than solvent-separated “peaks”. It is primarily a preparative technique; higher product concentration, higher purity, and increased throughput may be obtained compared to other modes of chromatography.

Spin column-based nucleic acid purification is a solid phase extraction method to quickly purify nucleic acids. This method relies on the fact that nucleic acid will bind to the solid phase of silica under certain conditions.
A monolithic HPLC column, or monolithic column, is a column used in high-performance liquid chromatography (HPLC). The internal structure of the monolithic column is created in such a way that many channels form inside the column. The material inside the column which separates the channels can be porous and functionalized. In contrast, most HPLC configurations use particulate packed columns; in these configurations, tiny beads of an inert substance, typically a modified silica, are used inside the column. Monolithic columns can be broken down into two categories, silica-based and polymer-based monoliths. Silica-based monoliths are known for their efficiency in separating smaller molecules while, polymer-based are known for separating large protein molecules.
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures. In other words, it's a scientific process of distinguishing to two or more substance in order to obtain purity. At least one product mixture of the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.
Perstraction is a membrane extraction process, where two solid phases are contacted across a membrane. The desired species in the feed, selectively crosses the membrane into the extracting solution. Perstraction was originally developed to overcome the downsides of liquid–liquid extraction, for example extractant toxicity and emulsion formation. Perstraction, or membrane extraction, has been applied to many fields including fermentation, waste water treatment and alcohol-free beverage production.
Industrial separation processes are technical procedures which are used in industry to separate a product from impurities or other products. The original mixture may either be a natural resource or the product of a chemical reaction.
Industrial enzymes are enzymes that are commercially used in a variety of industries such as pharmaceuticals, chemical production, biofuels, food & beverage, and consumer products. Due to advancements in recent years, biocatalysis through isolated enzymes is considered more economical than use of whole cells. Enzymes may be used as a unit operation within a process to generate a desired product, or may be the product of interest. Industrial biological catalysis through enzymes has experienced rapid growth in recent years due to their ability to operate at mild conditions, and exceptional chiral and positional specificity, things that traditional chemical processes lack. Isolated enzymes are typically used in hydrolytic and isomerization reactions. Whole cells are typically used when a reaction requires a co-factor. Although co-factors may be generated in vitro, it is typically more cost-effective to use metabolically active cells.




