
Pharmacology is a branch of medicine, biology, and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. It is the science of drugs including their origin, composition, pharmacokinetics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medicines. It is a miscellaneous science as it links health sciences with pharmaceutical sciences and natural sciences. The professional practice is becoming more clinically oriented as most of the drugs are now manufactured by pharmaceutical industries. Based on the setting, pharmacy practice is either classified as community or institutional pharmacy. Providing direct patient care in the community of institutional pharmacies is considered clinical pharmacy.
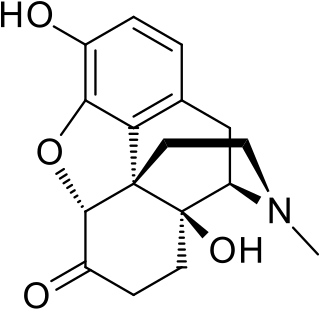
Oxymorphone is a highly potent opioid analgesic indicated for treatment of severe pain. Pain relief after injection begins after about 5–10 minutes, after oral administration it begins after about 30 minutes, and lasts about 3–4 hours for immediate-release tablets and 12 hours for extended-release tablets. The elimination half-life of oxymorphone is much faster intravenously, and as such, the drug is most commonly used orally. Like oxycodone, which metabolizes to oxymorphone, oxymorphone has a high potential to be abused.

Aniracetam, also known as N-anisoyl-2-pyrrolidinone, is a racetam which is sold in Europe as a prescription drug. It is not approved by the Food and Drug Administration for use in the United States as a prescription medication or dietary supplement. Despite the FDA's lack of approval, the drug is readily available over-the-counter in misbranded dietary supplements.

Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.

Cefalexin, also spelled cephalexin, is an antibiotic that can treat a number of bacterial infections. It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall. Cefalexin is a beta-lactam antibiotic within the class of first-generation cephalosporins. It works similarly to other agents within this class, including intravenous cefazolin, but can be taken by mouth.

Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to drug preparation, route of administration, site-specific targeting, metabolism, and toxicity are used to optimize efficacy and safety, and to improve patient convenience and compliance. Drug delivery is aimed at altering a drug's pharmacokinetics and specificity by formulating it with different excipients, drug carriers, and medical devices. There is additional emphasis on increasing the bioavailability and duration of action of a drug to improve therapeutic outcomes. Some research has also been focused on improving safety for the person administering the medication. For example, several types of microneedle patches have been developed for administering vaccines and other medications to reduce the risk of needlestick injury.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.

Critical Reviews in Microbiology is an international, peer-reviewed academic journal that publishes comprehensive review articles covering all areas of medical microbiology. Areas covered by the journal include bacteriology, virology, microbial genetics, epidemiology, and diagnostic microbiology. It is published by Taylor and Francis Group.

Critical Reviews in Biotechnology is an academic journal that publishes comprehensive review articles that organize, evaluate and present the current status of issues in biotechnology.

Established in 1972, Drug Metabolism Reviews is an academic journal that publishes review articles on all aspects of drug metabolism research. It is the official journal of the International Society for the Study of Xenobiotics (ISSX).
PK/PD modeling is a technique that combines the two classical pharmacologic disciplines of pharmacokinetics and pharmacodynamics. It integrates a pharmacokinetic and a pharmacodynamic model component into one set of mathematical expressions that allows the description of the time course of effect intensity in response to administration of a drug dose. PK/PD modeling is related to the field of pharmacometrics.

Terbogrel (INN) is an experimental drug that has been studied for its potential to prevent the vasoconstricting and platelet-aggregating action of thromboxanes. Terbogrel is an orally available thromboxane A2 receptor antagonist and a thromboxane A synthase inhibitor. The drug was developed by Boehringer Ingelheim.

Aripiprazole lauroxil, sold under the brand name Aristada, is a long-acting injectable atypical antipsychotic that was developed by Alkermes. It is an N-acyloxymethyl prodrug of aripiprazole that is administered via intramuscular injection once every four to eight weeks for the treatment of schizophrenia. Aripiprazole lauroxil was approved by the U.S. Food and Drug Administration (FDA) on 5 October 2015.

Chinedum Peace BabalolaFAS, FAAS is a Nigerian Professor of Pharmaceutical chemistry and Pharmacokinetics. She is the first female Professor of Pharmacy in the University of Ibadan, FAS and FAAS and the second female Nigerian FAAS. She is the incumbent Vice Chancellor of Chrisland University, Nigeria.
Leon Aarons is an Australian pharmacist who researches and teaches in the areas of pharmacodynamics and pharmacokinetics. He lives in the United Kingdom and from 1976 has been a professor of pharmacometrics at the University of Manchester. In the interest of promoting the effective development of drugs, the main focus of his work is optimizing pharmacological models, the design of clinical studies, and data analysis and interpretation in the field of population pharmacokinetics. From 1985 to 2010 Aarons was an editor emeritus of the Journal of Pharmacokinetics and Pharmacodynamics and is a former executive editor of the British Journal of Clinical Pharmacology.
A chiral switch is a chiral drug that has already approved as racemate but has been re-developed as a single enantiomer. The term chiral switching was introduced by Agranat and Caner in 1999 to describe the development of single enantiomers from racemate drugs. For example, levofloxacin is a chiral switch of racemic ofloxacin. The essential principle of a chiral switch is that there is a change in the status of chirality. In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention. A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties. To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been coined eutomer and distomer. The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred to as distomer. The eutomer/distomer ratio is called the eudisimic ratio and reflects the degree of enantioselectivity of the biological activity.
Malcolm Rowland FBPhS is Emeritus Professor of Pharmacy, University of Manchester, and Adjunct Professor, University of California San Francisco. His research in pharmacology, has been particularly in physiologically based pharmacokinetics. He has written several textbooks on the subject.
Kishor M. Wasan is a Canadian pharmacologist, pharmacist and professor. He was the dean of the University of Saskatchewan's College of Pharmacy and Nutrition from 2014 to 2019 and associate dean of research and graduate studies at the Faculty of Pharmaceutical Sciences at the University of British Columbia (UBC) from 2011 to 2014. Previously at UBC, he was chair of pharmaceutics and national director of the Canadian Summer Student Research Program after first joining the faculty in 1995. Wasan's research focuses on lipid-based drug delivery and the interaction between lipoprotein and pharmaceuticals. He has published more than 550 peer-reviewed articles and abstracts. He is a founding member and co-director of UBC's Neglected Global Diseases Initiative.













