
Fluorescence is one of two kinds of emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops.

In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Generally, fluorescent tagging, or labeling, uses a reactive derivative of a fluorescent molecule known as a fluorophore. The fluorophore selectively binds to a specific region or functional group on the target molecule and can be attached chemically or biologically. Various labeling techniques such as enzymatic labeling, protein labeling, and genetic labeling are widely utilized. Ethidium bromide, fluorescein and green fluorescent protein are common tags. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target.

Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications.

Fluorescence spectroscopy is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are rhodamine 6G, rhodamine 123, and rhodamine B. They are mainly used to dye paper and inks, but they lack the lightfastness for fabric dyeing.

A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image.
The Alexa Fluor family of fluorescent dyes is a series of dyes invented by Molecular Probes, now a part of Thermo Fisher Scientific, and sold under the Invitrogen brand name. Alexa Fluor dyes are frequently used as cell and tissue labels in fluorescence microscopy and cell biology. Alexa Fluor dyes can be conjugated directly to primary antibodies or to secondary antibodies to amplify signal and sensitivity or other biomolecules.

Two-photon excitation microscopy is a fluorescence imaging technique that is particularly well-suited to image scattering living tissue of up to about one millimeter in thickness. Unlike traditional fluorescence microscopy, where the excitation wavelength is shorter than the emission wavelength, two-photon excitation requires simultaneous excitation by two photons with longer wavelength than the emitted light. The laser is focused onto a specific location in the tissue and scanned across the sample to sequentially produce the image. Due to the non-linearity of two-photon excitation, mainly fluorophores in the micrometer-sized focus of the laser beam are excited, which results in the spatial resolution of the image. This contrasts with confocal microscopy, where the spatial resolution is produced by the interaction of excitation focus and the confined detection with a pinhole.

Fluorescein isothiocyanate (FITC) is a derivative of fluorescein used in wide-ranging applications including flow cytometry. First described in 1942, FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group (−N=C=S), replacing a hydrogen atom on the bottom ring of the structure. It is typically available as a mixture of isomers, fluorescein 5-isothiocyanate (5-FITC) and fluorescein 6-isothiocyanate (6-FITC). FITC is reactive towards nucleophiles including amine and sulfhydryl groups on proteins. It was synthesized by Robert Seiwald and Joseph Burckhalter in 1958.

Texas Red or sulforhodamine 101 acid chloride is a red fluorescent dye, used in histology for staining cell specimens, for sorting cells with fluorescent-activated cell sorting machines, in fluorescence microscopy applications, and in immunohistochemistry. Texas Red fluoresces at about 615 nm, and the peak of its absorption spectrum is at 589 nm. The powder is dark purple. Solutions can be excited by a dye laser tuned to 595-605 nm, or less efficiently a krypton laser at 567 nm. The absorption extinction coefficient at 596 nm is about 85,000 M−1cm−1.

In optics, photobleaching is the photochemical alteration of a dye or a fluorophore molecule such that it is permanently unable to fluoresce. This is caused by cleaving of covalent bonds or non-specific reactions between the fluorophore and surrounding molecules. Such irreversible modifications in covalent bonds are caused by transition from a singlet state to the triplet state of the fluorophores. The number of excitation cycles to achieve full bleaching varies. In microscopy, photobleaching may complicate the observation of fluorescent molecules, since they will eventually be destroyed by the light exposure necessary to stimulate them into fluorescing. This is especially problematic in time-lapse microscopy.
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV.
In chemistry, a dark quencher is a substance that absorbs excitation energy from a fluorophore and dissipates the energy as heat; while a typical (fluorescent) quencher re-emits much of this energy as light. Dark quenchers are used in molecular biology in conjunction with fluorophores. When the two are close together, such as in a molecule or protein, the fluorophore's emission is suppressed. This effect can be used to study molecular geometry and motion.

Vertico spatially modulated illumination (Vertico-SMI) is the fastest light microscope for the 3D analysis of complete cells in the nanometer range. It is based on two technologies developed in 1996, SMI and SPDM. The effective optical resolution of this optical nanoscope has reached the vicinity of 5 nm in 2D and 40 nm in 3D, greatly surpassing the λ/2 resolution limit applying to standard microscopy using transmission or reflection of natural light according to the Abbe resolution limit That limit had been determined by Ernst Abbe in 1873 and governs the achievable resolution limit of microscopes using conventional techniques.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. The intrinsic DNA fluorescence is very weak.Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
The FluoProbes series of fluorescent dyes were developed by Interchim to improve performances of standard fluorophores. They are designed for labeling biomolecules, cells, tissues or beads in advanced fluorescent detection techniques.
Time-resolved fluorescence energy transfer (TR-FRET) is the practical combination of time-resolved fluorometry (TRF) with Förster resonance energy transfer (FRET) that offers a powerful tool for drug discovery researchers. TR-FRET combines the low background aspect of TRF with the homogeneous assay format of FRET. The resulting assay provides an increase in flexibility, reliability and sensitivity in addition to higher throughput and fewer false positive/false negative results. FRET involves two fluorophores, a donor and an acceptor. Excitation of the donor by an energy source produces an energy transfer to the acceptor if the two are within a given proximity to each other. The acceptor in turn emits light at its characteristic wavelength.

Pacific Blue, or systematically 3-carboxy-6,8-difluoro-7-hydroxycoumarin, is a fluorophore used in cell biology. Its excitation maximum lies at 401 nm, while its emission maximum is at 452 nm. In contrast to the less acidic 7-hydroxy-3-carboxycoumarin (pKa=7.0), the high acidity of the phenol of Pacific Blue (pKa=3.7) causes its fluorescence to remain very high at neutral pH.
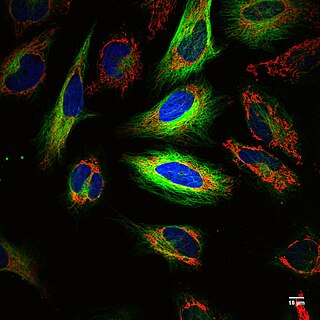
Fluorescence imaging is a type of non-invasive imaging technique that can help visualize biological processes taking place in a living organism. Images can be produced from a variety of methods including: microscopy, imaging probes, and spectroscopy.














