
Progesterone is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.
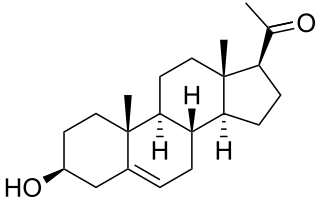
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Dexanabinol is a synthetic cannabinoid derivative in development by e-Therapeutics plc. It is the "unnatural" enantiomer of the potent cannabinoid agonist HU-210. Unlike other cannabinoid derivatives, HU-211 does not act as a cannabinoid receptor agonist, but instead as an NMDA antagonist. It therefore does not produce cannabis-like effects, but is anticonvulsant and neuroprotective, and is widely used in scientific research as well as currently being studied for applications such as treating head injury, stroke, or cancer. It was shown to be safe in clinical trials and is currently undergoing Phase I trials for the treatment of brain cancer and advanced solid tumors.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Progesterone (P4), sold under the brand name Prometrium among others, is a medication and naturally occurring steroid hormone. It is a progestogen and is used in combination with estrogens mainly in hormone therapy for menopausal symptoms and low sex hormone levels in women. It is also used in women to support pregnancy and fertility and to treat gynecological disorders. Progesterone can be taken by mouth, vaginally, and by injection into muscle or fat, among other routes. A progesterone vaginal ring and progesterone intrauterine device used for birth control also exist in some areas of the world.

P1-185, also known as progesterone 3-O-(L-valine)-E-oxime or as pregn-4-ene-3,20-dione 3-O-(L-valine)-E-oxime, is a synthetic progestogen and neurosteroid and an oxime ester analogue and prodrug of progesterone. It was developed as an improved water-soluble version of progesterone such that it could be formulated as an aqueous preparation and easily and rapidly administered intravenously as a potential therapy for traumatic brain injury. However, the chemical synthesis of P1-185 was described as somewhat challenging, so oxime conjugates of progesterone of the C20 instead of C3 position, such as EIDD-1723 and EIDD-036, have since been developed.

Pregnenolone succinate is a synthetic pregnane steroid and an ester of pregnenolone which is described as a glucocorticoid and anti-inflammatory drug and has been patented and marketed as a topical medication in the form of a cream for the treatment of allergic, pruritic, and inflammatory dermatitis. It has also been described as a non-hormonal sterol, having neurosteroid activity, and forming a progesterone analogue via dehydrogenation.

BNN-20, also known as 17β-spiro-(androst-5-en-17,2'-oxiran)-3β-ol, is a synthetic neurosteroid, "microneurotrophin", and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA, TrkB, and p75NTR, receptors for the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), as well as for DHEA and DHEA sulfate (DHEA-S). The drug has been suggested as a potential novel treatment for Parkinson's disease and other conditions.

BNN-27, also known as 17α,20R-epoxypregn-5-ene-3β,21-diol, is a synthetic neurosteroid and "microneurotrophin" and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA and p75NTR, receptors for nerve growth factor (NGF) and other neurotrophins, as well as for DHEA and DHEA sulfate (DHEA-S). BNN-27 has neuroprotective and neurogenic effects and has been suggested as a potential novel treatment for neurodegenerative diseases and brain trauma.

EIDD-1723, also known as EPRX-01723 or as progesterone 20E-[O-[(phosphonooxy)methyl]oxime] sodium salt, is a synthetic, water-soluble analogue of progesterone and a neurosteroid which was developed for the potential treatment of traumatic brain injury. It is a rapidly converted prodrug of EIDD-036, which is considered to be the active form of the agent. Previous C3 and C20 oxime derivatives of progesterone, such as P1-185, were also developed and studied prior to EIDD-1723.
VOLT-02 is a water-soluble conjugate of progesterone and a neurosteroid which is under development by Levolta Pharmaceuticals for the treatment of traumatic brain injury, gynecological disorders, and menstrual disorders. As of March 2017, it is in phase II clinical trials for these indications. The chemical structure of VOLT-02 does not appear to have been released yet.

Progesterone carboxymethyloxime, or progesterone 3-(O-carboxymethyl)oxime (P4-3-CMO), is a progestin which was never marketed. It is an oral prodrug of progesterone with improved pharmacokinetic properties. The compound was developed in an attempt to address the poor oral pharmacokinetics of progesterone, including its very low bioavailability and short biological half-life. These properties of progesterone are thought to be caused by its low water solubility and high metabolic clearance rate due to rapid degradation in the intestines and liver. Drugs with low aqueous solubility are not absorbed well in the intestines because their dissolution in water is limited.
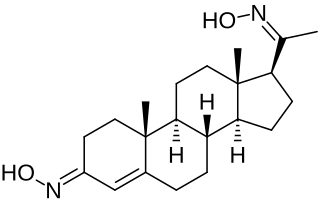
Progesterone dioxime, or progesterone 3,20-dioxime (P4-3,20-DO), also known as 3,20-di(hydroxyimino)pregn-4-en-3-one, is a progesterone derivative which was never marketed. It is a progestogen oxime – specifically, the C3 and C20 dioxime of the progestogen progesterone. Progesterone C3 and C20 oxime conjugates have been found to be water-soluble prodrugs of progesterone and pregnane neurosteroids.

Progesterone 3-oxime (P4-3-O), also known as 3-(hydroxyimino)pregn-4-en-3-one, is a progesterone derivative which was never marketed. It is a progestogen oxime – specifically, the C3 oxime of the progestogen progesterone. Progesterone C3 and C20 oxime conjugates, like progesterone 3-(O-carboxymethyl)oxime, have been found to be water-soluble prodrugs of progesterone and pregnane neurosteroids.

Pregnenolone, sold under the brand name Enelone among others, is a medication and supplement as well as a naturally occurring and endogenous steroid. It is described as a neurosteroid and anti-inflammatory drug and was used in the treatment of rheumatoid arthritis and soft-tissue rheumatism in the 1950s and is no longer prescribed today, but remains available as a supplement. Pregnenolone can be taken by mouth, as a topical medication, or by injection into muscle.
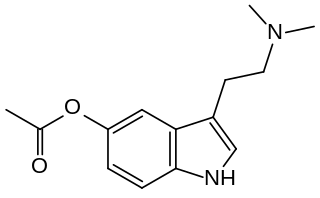
O-Acetylbufotenine, or bufotenine O-acetate, also known as 5-acetoxy-N,N-dimethyltryptamine (5-AcO-DMT) or O-acetyl-N,N-dimethylserotonin, is a synthetic tryptamine derivative and putative serotonergic psychedelic. It is the O-acetylated analogue of the naturally occurring peripherally selective serotonergic tryptamine bufotenine and is thought to act as a centrally penetrant prodrug of bufotenine.















