
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.

Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces.

The contact angle is the angle, conventionally measured through the liquid, where a liquid–vapor interface meets a solid surface. It quantifies the wettability of a solid surface by a liquid via the Young equation. A given system of solid, liquid, and vapor at a given temperature and pressure has a unique equilibrium contact angle. However, in practice a dynamic phenomenon of contact angle hysteresis is often observed, ranging from the advancing (maximal) contact angle to the receding (minimal) contact angle. The equilibrium contact is within those values, and can be calculated from them. The equilibrium contact angle reflects the relative strength of the liquid, solid, and vapour molecular interaction.

Cassie's law, or the Cassie equation, describes the effective contact angle θc for a liquid on a chemically heterogeneous surface, i.e. the surface of a composite material consisting of different chemistries, that is non uniform throughout. Contact angles are important as they quantify a surface's wettability, the nature of solid-fluid intermolecular interactions. Cassie's law is reserved for when a liquid completely covers both smooth and rough heterogeneous surfaces.

Ultrahydrophobic surfaces are highly hydrophobic, i.e., extremely difficult to wet. The contact angles of a water droplet on an ultrahydrophobic material exceed 150°. This is also referred to as the lotus effect, after the superhydrophobic leaves of the lotus plant. A droplet striking these kinds of surfaces can fully rebound like an elastic ball. Interactions of bouncing drops can be further reduced using special superhydrophobic surfaces that promote symmetry breaking, pancake bouncing or waterbowl bouncing.

In physics, a "coffee ring" is a pattern left by a puddle of particle-laden liquid after it evaporates. The phenomenon is named for the characteristic ring-like deposit along the perimeter of a spill of coffee. It is also commonly seen after spilling red wine. The mechanism behind the formation of these and similar rings is known as the coffee ring effect or in some instances, the coffee stain effect, or simply ring stain.
The Vroman effect, named after Leo Vroman, describes the process of competitive protein adsorption to a surface by blood serum proteins. The highest mobility proteins generally arrive first and are later replaced by less mobile proteins that have a higher affinity for the surface. The order of protein adsorption also depends on the molecular weight of the species adsorbing. Typically, low molecular weight proteins are displaced by high molecular weight protein while the opposite, high molecular weight being displaced by low molecular weight, does not occur. A typical example of this occurs when fibrinogen displaces earlier adsorbed proteins on a biopolymer surface and is later replaced by high molecular weight kininogen. The process is delayed in narrow spaces and on hydrophobic surfaces, fibrinogen is usually not displaced. Under stagnant conditions initial protein deposition takes place in the sequence: albumin; globulin; fibrinogen; fibronectin; factor XII, and HMWK.
Small-angle X-ray scattering (SAXS) is a small-angle scattering technique by which nanoscale density differences in a sample can be quantified. This means that it can determine nanoparticle size distributions, resolve the size and shape of (monodisperse) macromolecules, determine pore sizes, characteristic distances of partially ordered materials, and much more. This is achieved by analyzing the elastic scattering behaviour of X-rays when travelling through the material, recording their scattering at small angles. It belongs to the family of small-angle scattering (SAS) techniques along with small-angle neutron scattering, and is typically done using hard X-rays with a wavelength of 0.07 – 0.2 nm.. Depending on the angular range in which a clear scattering signal can be recorded, SAXS is capable of delivering structural information of dimensions between 1 and 100 nm, and of repeat distances in partially ordered systems of up to 150 nm. USAXS can resolve even larger dimensions, as the smaller the recorded angle, the larger the object dimensions that are probed.

The ouzo effect is a milky oil-in-water emulsion that is formed when water is added to ouzo and other anise-flavored liqueurs and spirits, such as pastis, rakı, arak, sambuca and absinthe. Such emulsions occur with only minimal mixing and are highly stable.
A Pickering emulsion is an emulsion that is stabilized by solid particles which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903.
A wetting transition may occur during the process of wetting of a solid surface with a liquid. The transition corresponds to a certain change in contact angle, the macroscopic parameter characterizing wetting. Various contact angles can co-exist on the same solid substrate. Wetting transitions may occur in a different way depending on whether the surface is flat or rough.
Undecylic acid (systematically named undecanoic acid) is a carboxylic acid with chemical formula CH3(CH2)9COOH. It is often used as an antifungal agent, to treat ringworm and athlete's foot, for example. Like decanoic acid, it has a distinctive, unpleasant odor.
A slippery liquid-infused porous surface (SLIPS), liquid-impregnated surface (LIS), or multi-phase surface consists of two distinct layers. The first is a highly textured or porous substrate with features spaced sufficiently close to stably contain the second layer which is an impregnating liquid that fills in the spaces between the features. The liquid must have a surface energy well-matched to the substrate in order to form a stable film. Slippery surfaces are finding applications in commercial products, anti-fouling surfaces, anti-icing and biofilm-resistant medical devices.
Self-cleaning surfaces are a class of materials with the inherent ability to remove any debris or bacteria from their surfaces in a variety of ways. The self-cleaning functionality of these surfaces are commonly inspired by natural phenomena observed in lotus leaves, gecko feet, and water striders to name a few. The majority of self-cleaning surfaces can be placed into three categories:
- superhydrophobic
- superhydrophilic
- photocatalytic.

Liquid marbles are non-stick droplets wrapped by micro- or nano-metrically scaled hydrophobic, colloidal particles ; representing a platform for a diversity of chemical and biological applications. Liquid marbles are also found naturally; aphids convert honeydew droplets into marbles. A variety of non-organic and organic liquids may be converted into liquid marbles. Liquid marbles demonstrate elastic properties and do not coalesce when bounced or pressed lightly. Liquid marbles demonstrate a potential as micro-reactors, micro-containers for growing micro-organisms and cells, micro-fluidics devices, and have even been used in unconventional computing. Liquid marbles remain stable on solid and liquid surfaces. Statics and dynamics of rolling and bouncing of liquid marbles were reported. Liquid marbles coated with poly-disperse and mono-disperse particles have been reported. Liquid marbles are not hermetically coated by solid particles but connected to the gaseous phase. Kinetics of the evaporation of liquid marbles has been investigated.
Self-propulsion is the autonomous displacement of nano-, micro- and macroscopic natural and artificial objects, containing their own means of motion. Self-propulsion is driven mainly by interfacial phenomena. Various mechanisms of self-propelling have been introduced and investigated, which exploited phoretic effects, gradient surfaces, breaking the wetting symmetry of a droplet on a surface, the Leidenfrost effect, the self-generated hydrodynamic and chemical fields originating from the geometrical confinements, and soluto- and thermo-capillary Marangoni flows. Self-propelled system demonstrate a potential as micro-fluidics devices and micro-mixers. Self-propelled liquid marbles have been demonstrated.
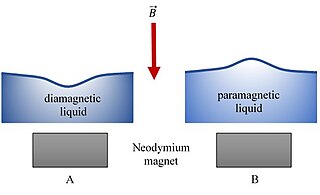
In physics, the Moses effect is a phenomenon of deformation of the surface of a diamagnetic liquid by a magnetic field. The effect was named after the biblical figure Moses, inspired by the mythological crossing of the Red Sea in the Old Testament.
Interfacial rheology is a branch of rheology that studies the flow of matter at the interface between a gas and a liquid or at the interface between two immiscible liquids. The measurement is done while having surfactants, nanoparticles or other surface active compounds present at the interface. Unlike in bulk rheology, the deformation of the bulk phase is not of interest in interfacial rheology and its effect is aimed to be minimized. Instead, the flow of the surface active compounds is of interest.
Chiara Neto is an Italian Australian chemist and Professor of Physical Chemistry at the University of Sydney. Her research considers functional nanostructures and the design of new materials for sustainable technologies. She is the former President of the Australasian Colloid and Interface Society and was selected as an Australian Research Council Future Fellow in 2018.
Stefan A. F. Bon is a Professor of Chemical Engineering in the department of Chemistry at the University of Warwick, United Kingdom. His research considers polymer-based colloids. He is a Fellow of the International Union of Pure and Applied Chemistry, an elected member of the International Polymer Colloids Group (IPCG), and member of the physical Newton international fellowship committee, and served as the Royal Society of Chemistry Outreach Lecturer in 2015-2016.









