
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials (chemicals) depending on the type of battery.

In electrochemistry, cyclic voltammetry (CV) is a type of voltammetric measurement where the potential of the working electrode is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles in potential are repeated until the voltammetric trace reaches a cyclic steady state. The current at the working electrode is plotted versus the voltage at the working electrode to yield the cyclic voltammogram. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

A quartz crystal microbalance (QCM) measures a mass variation per unit area by measuring the change in frequency of a quartz crystal resonator. The resonance is disturbed by the addition or removal of a small mass due to oxide growth/decay or film deposition at the surface of the acoustic resonator. The QCM can be used under vacuum, in gas phase and more recently in liquid environments. It is useful for monitoring the rate of deposition in thin-film deposition systems under vacuum. In liquid, it is highly effective at determining the affinity of molecules to surfaces functionalized with recognition sites. Larger entities such as viruses or polymers are investigated as well. QCM has also been used to investigate interactions between biomolecules. Frequency measurements are easily made to high precision ; hence, it is easy to measure mass densities down to a level of below 1 μg/cm2. In addition to measuring the frequency, the dissipation factor is often measured to help analysis. The dissipation factor is the inverse quality factor of the resonance, Q−1 = w/fr ; it quantifies the damping in the system and is related to the sample's viscoelastic properties.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.

Electrogravimetry is a method used to separate and quantify ions of a substance, usually a metal. In this process, the analyte solution is electrolyzed. Electrochemical reduction causes the analyte to be deposited on the cathode. The mass of the cathode is determined before and after the experiment, and the difference is used to calculate the mass of analyte in the original solution. Controlling the potential of the electrode is important to ensure that only the metal being analyzed will be deposited on the electrode.
The Sauerbrey equation was developed by the German Günter Sauerbrey in 1959, while working on his doctoral thesis at Technische Universität Berlin, Germany. It is a method for correlating changes in the oscillation frequency of a piezoelectric crystal with the mass deposited on it. He simultaneously developed a method for measuring the characteristic frequency and its changes by using the crystal as the frequency determining component of an oscillator circuit. His method continues to be used as the primary tool in quartz crystal microbalance (QCM) experiments for conversion of frequency to mass and is valid in nearly all applications.
A polymer-based battery uses organic materials instead of bulk metals to form a battery. Currently accepted metal-based batteries pose many challenges due to limited resources, negative environmental impact, and the approaching limit of progress. Redox active polymers are attractive options for electrodes in batteries due to their synthetic availability, high-capacity, flexibility, light weight, low cost, and low toxicity. Recent studies have explored how to increase efficiency and reduce challenges to push polymeric active materials further towards practicality in batteries. Many types of polymers are being explored, including conductive, non-conductive, and radical polymers. Batteries with a combination of electrodes are easier to test and compare to current metal-based batteries, however batteries with both a polymer cathode and anode are also a current research focus. Polymer-based batteries, including metal/polymer electrode combinations, should be distinguished from metal-polymer batteries, such as a lithium polymer battery, which most often involve a polymeric electrolyte, as opposed to polymeric active materials.
Heinz Gerischer was a German chemist who specialized in electrochemistry. He was the thesis advisor of future Nobel laureate Gerhard Ertl.
In electrochemistry, ITIES is an electrochemical interface that is either polarisable or polarised. An ITIES is polarisable if one can change the Galvani potential difference, or in other words the difference of inner potentials between the two adjacent phases, without noticeably changing the chemical composition of the respective phases. An ITIES system is polarised if the distribution of the different charges and redox species between the two phases determines the Galvani potential difference.
Within surface science, a quartz crystal microbalance with dissipation monitoring (QCM-D) is a type of quartz crystal microbalance (QCM) based on the ring-down technique. It is used in interfacial acoustic sensing. Its most common application is the determination of a film thickness in a liquid environment. It can be used to investigate further properties of the sample, most notably the layer's softness.
Water oxidation is one of the half reactions of water splitting:
Transport Number is the ratio of the current carried by a given ionic species through a cross section of an electrolytic solution to the total current passing through the cross section. Differences in transport number arise from differences in electrical mobility. For example, in an aqueous solution of sodium chloride, less than half of the current is carried by the positively charged sodium ions (cations) and more than half is carried by the negatively charged chloride ions (anions) because the chloride ions are able to move faster, i.e., chloride ions have higher mobility than sodium ions. The sum of the transport numbers for all of the ions in solution always equals unity:

Bipolar electrochemistry is a phenomenon in electrochemistry based on the polarization of conducting objects in electric fields. Indeed, this polarization generates a potential difference between the two extremities of the substrate that is equal to the electric field value multiplied by the size of the object. If this potential difference is important enough, then redox reactions can be generated at the extremities of the object, oxidations will occur at one extremity coupled simultaneously to reductions at the other extremity. In a simple experimental setup consisting of a platinum wire in a weighing boat containing a pH indicator solution, a 30 V voltage across two electrodes will cause water reduction at one end of the wire and a pH increase and water oxidation at the anodic end and a pH decrease. The poles of the bipolar electrode also align themselves with the applied electric field.
Scanning electrochemical microscopy (SECM) is a technique within the broader class of scanning probe microscopy (SPM) that is used to measure the local electrochemical behavior of liquid/solid, liquid/gas and liquid/liquid interfaces. Initial characterization of the technique was credited to University of Texas electrochemist, Allen J. Bard, in 1989. Since then, the theoretical underpinnings have matured to allow widespread use of the technique in chemistry, biology and materials science. Spatially resolved electrochemical signals can be acquired by measuring the current at an ultramicroelectrode (UME) tip as a function of precise tip position over a substrate region of interest. Interpretation of the SECM signal is based on the concept of diffusion-limited current. Two-dimensional raster scan information can be compiled to generate images of surface reactivity and chemical kinetics.
A biotransducer is the recognition-transduction component of a biosensor system. It consists of two intimately coupled parts; a bio-recognition layer and a physicochemical transducer, which acting together converts a biochemical signal to an electronic or optical signal. The bio-recognition layer typically contains an enzyme or another binding protein such as antibody. However, oligonucleotide sequences, sub-cellular fragments such as organelles and receptor carrying fragments, single whole cells, small numbers of cells on synthetic scaffolds, or thin slices of animal or plant tissues, may also comprise the bio-recognition layer. It gives the biosensor selectivity and specificity. The physicochemical transducer is typically in intimate and controlled contact with the recognition layer. As a result of the presence and biochemical action of the analyte, a physico-chemical change is produced within the biorecognition layer that is measured by the physicochemical transducer producing a signal that is proportionate to the concentration of the analyte. The physicochemical transducer may be electrochemical, optical, electronic, gravimetric, pyroelectric or piezoelectric. Based on the type of biotransducer, biosensors can be classified as shown to the right.
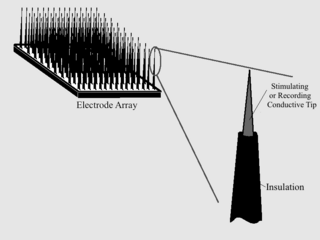
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.

Pseudocapacitance is the electrochemical storage of electricity in an electrochemical capacitor that occurs due to faradaic charge transfer originating from a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes. Pseudocapacitance is accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion. One electron per charge unit is involved. The adsorbed ion has no chemical reaction with the atoms of the electrode since only a charge-transfer takes place. Supercapacitors that rely primarily on pseudocapacitance are sometimes called pseudocapacitors.
Electrochemical AFM (EC-AFM) is a particular type of Scanning probe microscopy (SPM), which combines the classical Atomic force microscopy (AFM) together with electrochemical measurements. EC-AFM allows to perform in-situ AFM measurements in an electrochemical cell, in order to investigate the actual changes in the electrode surface morphology during electrochemical reactions. The solid-liquid interface is thus investigated. This technique was developed for the first time in 1996 by Kouzeki et al., who studied amorphous and polycrystalline thin films of Naphthalocyanine on Indium tin oxide in a solution of 0.1 M Potassium chloride (KCl). Unlike the Electrochemical scanning tunneling microscope, previously developed by Itaya and Tomita in 1988, the tip is non-conductive and it is easily steered in a liquid environment.
Calcium (ion) batteries are energy storage and delivery technologies (i.e., electro–chemical energy storage) that employ calcium ions (cations), Ca2+, as the active charge carrier. Calcium (ion) batteries remain an active area of research, with studies and work persisting in the discovery and development of electrodes and electrolytes that enable stable, long-term battery operation. Calcium batteries are rapidly emerging as a recognized alternative to Li-ion technology due to their similar performance, significantly greater abundance, and lower cost.






















