Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Vegetable oils, or vegetable fats, are oils extracted from seeds or from other parts of edible plants. Like animal fats, vegetable fats are mixtures of triglycerides. Soybean oil, grape seed oil, and cocoa butter are examples of seed oils, or fats from seeds. Olive oil, palm oil, and rice bran oil are examples of fats from other parts of plants. In common usage, vegetable oil may refer exclusively to vegetable fats which are liquid at room temperature. Vegetable oils are usually edible.

An essential oil is a concentrated hydrophobic liquid containing volatile chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does not mean indispensable or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism.
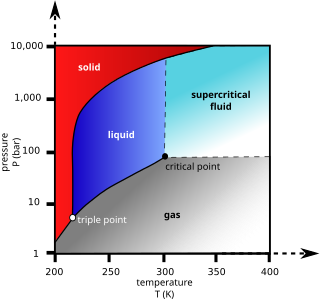
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCFs are superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned".
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This process renders a product (chemistry) and by-products.
Decaffeination is the removal ("de-") of caffeine from coffee beans, cocoa, tea leaves, and other caffeine-containing materials. Decaffeinated products are commonly termed by the abbreviation decaf. Decaffeinated drinks contain typically 1–2% of the original caffeine content, but sometimes as much as 20%.
Micronization is the process of reducing the average diameter of a solid material's particles. Traditional techniques for micronization focus on mechanical means, such as milling and grinding. Modern techniques make use of the properties of supercritical fluids and manipulate the principles of solubility.

Maceration is the winemaking process where the phenolic materials of the grape—tannins, coloring agents (anthocyanins) and flavor compounds—are leached from the grape skins, seeds and stems into the must. To macerate is to soften by soaking, and maceration is the process by which the red wine receives its red color, since raw grape juice is clear-grayish in color. In the production of white wines, maceration is either avoided or allowed only in very limited manner in the form of a short amount of skin contact with the juice prior to pressing. This is more common in the production of varietals with less natural flavor and body structure like Sauvignon blanc and Sémillon. For Rosé, red wine grapes are allowed some maceration between the skins and must, but not to the extent of red wine production.

Rose oil is the essential oil extracted from the petals of various types of rose. Rose ottos are extracted through steam distillation, while rose absolutes are obtained through solvent extraction, the absolute being used more commonly in perfumery. The production technique originated in Greater Iran. Even with their high price and the advent of organic synthesis, rose oils are still perhaps the most widely used essential oil in perfumery.
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.

The following outline is provided as an overview of and topical guide to the preparation of food:

Fragrance extraction refers to the separation process of aromatic compounds from raw materials, using methods such as distillation, solvent extraction, expression, sieving, or enfleurage. The results of the extracts are either essential oils, absolutes, concretes, or butters, depending on the amount of waxes in the extracted product.

Plant oils or vegetable oils are oils derived from plant sources, as opposed to animal fats or petroleum. There are three primary types of plant oil, differing both the means of extracting the relevant parts of the plant, and in the nature of the resulting oil:
- Vegetable fats and oils were historically extracted by putting part of the plant under pressure, squeezing out the oil.
- Macerated oils consist of a base oil to which parts of plants are added.
- Essential oils are composed of volatile aromatic compounds, extracted from plants by distillation.

Superheated water is liquid water under pressure at temperatures between the usual boiling point, 100 °C (212 °F) and the critical temperature, 374 °C (705 °F). It is also known as "subcritical water" or "pressurized hot water". Superheated water is stable because of overpressure that raises the boiling point, or by heating it in a sealed vessel with a headspace, where the liquid water is in equilibrium with vapour at the saturated vapor pressure. This is distinct from the use of the term superheating to refer to water at atmospheric pressure above its normal boiling point, which has not boiled due to a lack of nucleation sites.

Chicken fat is fat obtained from chicken rendering and processing. Of the many animal-sourced substances, chicken fat is noted for being high in linoleic acid, an omega-6 fatty acid. Linoleic acid levels are between 17.9% and 22.8%. It is a common flavoring, additive or main component of chicken soup. It is often used in pet foods, and has been used in the production of biodiesel. One method of converting chicken fat into biodiesel is through a process called supercritical methanol treatment.
Winterizationof oil is a process that uses a solvent and cold temperatures to separate lipids and other desired oil compounds from waxes. Winterization is a type of fractionation, the general process of separating the triglycerides found in fats and oils, using the difference in their melting points, solubility, and volatility.

In chemistry, recrystallization is a technique used to purify chemicals. By dissolving a mixture of a compound and impurities in an appropriate solvent, either the desired compound or impurities can be removed from the solution, leaving the other behind. It is named for the crystals often formed when the compound precipitates out. Alternatively, recrystallization can refer to the natural growth of larger ice crystals at the expense of smaller ones.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.

Cooking oil is a plant or animal liquid fat used in frying, baking, and other types of cooking. Oil allows higher cooking temperatures than water, making cooking faster and more flavorful, while likewise distributing heat, reducing burning and uneven cooking. It sometimes imparts its own flavor. Cooking oil is also used in food preparation and flavoring not involving heat, such as salad dressings and bread dips.

Hemp juice is a beverage derived from industrial hemp, made from the result of pressing the Cannabis sativa plant. The juice is obtained through a large-scale industrial cold-pressing procedure using the upper parts of the hemp plant as well as the leaves. This procedure distinguishes hemp juice from other hemp products such as hemp oil, hemp sprouts or hemp milk, which are obtained through the seeds of the hemp plant.










