
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of −CnH2n+1. A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula −CnH2n−1. Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula −CH3.
Acrylates are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion CH2=CHCO−2. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities.

Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH3CH2CH2COOCH2CH3. It is soluble in propylene glycol, paraffin oil, and kerosene. It has a fruity odor, similar to pineapple, and is a key ingredient used as a flavor enhancer in processed orange juices. It also occurs naturally in many fruits, albeit at lower concentrations.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
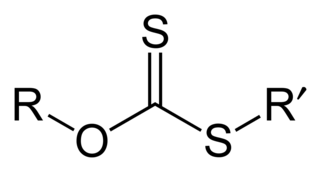
A xanthate is a salt or ester of a xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+. Xanthate also refers to the anion [R−O−CS2]−. The formula of a xanthic acid is R−O−C(=S)−S−H, such as ethyl xanthic acid, while the formula of an ester of a xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός (xanthos) meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.

Ethyl formate is an ester formed when ethanol reacts with formic acid. Ethyl formate has the characteristic smell of rum and is partially responsible for the flavor of raspberries, occuring naturally in some plant oils, fruits, and juices. Ethyl formate does not occur naturally in the animal kingdom.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate.

Methyl cellulose is a compound derived from cellulose. It is sold under a variety of trade names and is used as a thickener and emulsifier in various food and cosmetic products, and also as a bulk-forming laxative. Like cellulose, it is not digestible, non-toxic, and not an allergen. In addition to culinary uses, it is used in arts and crafts such as papier-mâché and is often the main ingredient of wallpaper paste.
Glycol ethers are a class of chemical compounds consisting of alkyl ethers that are based on glycols such as ethylene glycol or propylene glycol. They are commonly used as solvents in paints and cleaners. They have good solvent properties while having higher boiling points than the lower-molecular-weight ethers and alcohols.

Hypromellose (INN), short for hydroxypropyl methylcellulose (HPMC), is a semisynthetic, inert, viscoelastic polymer used in eye drops, as well as an excipient and controlled-delivery component in oral medicaments, found in a variety of commercial products.

Methylpentynol is a tertiary pentynol with hypnotic/sedative and anticonvulsant effects and an exceptionally low therapeutic index. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of insomnia, but its use was quickly phased out in response to newer drugs with far more favorable safety profiles.

Ethyl cellulose is a derivative of cellulose in which some of the hydroxyl groups on the repeating glucose units are converted into ethyl ether groups. The number of ethyl groups can vary depending on the manufacturer.

2-Hexanone is a ketone used as a general solvent and in paints. It dissolves cellulose nitrate, vinyl polymers and copolymers, and natural and synthetic resins. It is recommended as a solvent because it is photochemically inactive; however it has a very low safe threshold limit value. 2-Hexanone is absorbed through the lungs, orally and dermally and its metabolite, 2,5-hexanedione, is neurotoxic. Animal tests have shown that the neurotoxic effect of 2-hexanone may be potentiated by simultaneous administration of 2-butanone.

1-Ethyl-3-methylimidazolium chloride or [EMIM]Cl is an ionic liquid that can be used in cellulose processing. The cation consists of a five-membered ring with two nitrogen and three carbon atoms, i.e. a derivative of imidazole, with ethyl and methyl groups substituted at the two nitrogen atoms. Its melting point is 77–79 °C.
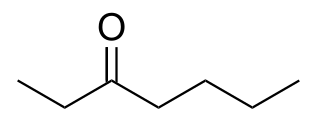
3-Heptanone, is a seven carbon ketone. It is a colorless liquid with a "green odor," also described to have a fruity scent. It is often used as a perfume/fragrance, as a solvent for cellulose, nitrocellulose, or vinyl resins, and as a synthetic building block in the preparation of other organic molecules.

Methyl lactate, also known as lactic acid methyl ester, is the organic compound with the formula CH3CH(OH)CO2CH3. It is the methyl ester of lactic acid. A colorless liquid, it is the simplest chiral ester. Being naturally derived, it is readily available as a single enantiomer.
















