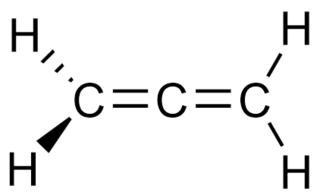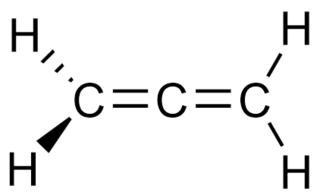
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. An group of the structure R2C=C=CR− is called allenyl, where R is H or some alkyl group. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Ethylene is a hydrocarbon which has the formula C2H4 or H2C=CH2. It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. Ethylene glycol has a sweet taste, but it is toxic in high concentrations. This molecule has been observed in outer space.

1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula C
2H
4Br
2. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint, sweet odor, detectable at 10 ppm, and is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive.

Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst.

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word synthon has now come to be used to mean synthetic building block rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book The Logic of Chemical Synthesis, as it was not included in the index. Because synthons are charged, when placed into a synthesis an uncharged form is found commercially instead of forming and using the potentially very unstable charged synthons.
In organic chemistry a halohydrin is a functional group in which a halogen and a hydroxyl are bonded to adjacent carbon atoms, which otherwise bear only hydrogen or hydrocarbyl groups. The term only applies to saturated motifs, as such compounds like 2-chlorophenol would not normally be considered halohydrins. Megatons of some chlorohydrins, e.g. propylene chlorohydrin, are produced annually as precursors to polymers.

Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Tetrathiafulvalene (TTF) is an organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.

Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid.
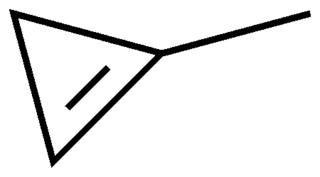
1-Methylcyclopropene (1-MCP) is a cyclopropene derivative used as a synthetic plant growth regulator. It is structurally related to the natural plant hormone ethylene and it is used commercially to slow down the ripening of fruit and to help maintain the freshness of cut flowers.

Carbon diselenide is an inorganic compound with the chemical formula CSe
2. It is a yellow-orange oily liquid with pungent odor. It is the selenium analogue of carbon disulfide and carbon dioxide. This light-sensitive compound is insoluble in water and soluble in organic solvents.
Organochromium chemistry is a branch of organometallic chemistry that deals with organic compounds containing a chromium to carbon bond and their reactions. The field is of some relevance to organic synthesis. The relevant oxidation states for organochromium complexes encompass the entire range of possible oxidation states from –4 (d10) in Na4[Cr–IV(CO)4] to +6 (d0) in oxo-alkyl complexes like Cp*CrVI(=O)2Me.

Thiocarbonic acid is an inorganic acid with the chemical formula H2CS3. It is an analog of carbonic acid H2CO3, in which all oxygen atoms are replaced with sulfur atoms. It is an unstable hydrophobic red oily liquid.

Sodium 1,3-dithiole-2-thione-4,5-dithiolate is the organosulfur compound with the formula Na2C3S5, abbreviated Na2dmit. It is the sodium salt of the conjugate base of the 1,3-dithiole-2-thione-4,5-dithiol. The salt is a precursor to dithiolene complexes and tetrathiafulvalenes.

Dimethyl trithiocarbonate is an organic compound with the chemical formula S=C(SCH3)2. It is a methyl ester of trithiocarbonic acid. This chemical belongs to a subcategory of esters called thioesters. It is a sulfur analog of dimethyl carbonate O=C(OCH3)2, where all three oxygen atoms are replaced with sulfur atoms. Dimethyl trithiocarbonate is a yellow liquid with a strong and unpleasant odor.