
In physics, the Maxwell–Boltzmann distribution is a particular probability distribution named after James Clerk Maxwell and Ludwig Boltzmann.
The molar gas constant is denoted by the symbol R or R. It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per mole, i.e. the pressure–volume product, rather than energy per temperature increment per particle. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation.
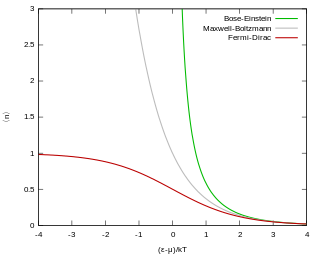
Fermi-Dirac statistics is a type of quantum statistics that applies to the physics of a system consisting of many identical particles that obey the Pauli exclusion principle. A result is the Fermi–Dirac distribution of particles over energy states. It is named after Enrico Fermi and Paul Dirac, each of whom derived the distribution independently in 1926. Fermi–Dirac statistics is a part of the field of statistical mechanics and uses the principles of quantum mechanics.

In mathematics and physics, the heat equation is a certain partial differential equation. Solutions of the heat equation are sometimes known as caloric functions. The theory of the heat equation was first developed by Joseph Fourier in 1822 for the purpose of modeling how a quantity such as heat diffuses through a given region.

An ideal Fermi gas is a state of matter which is an ensemble of many non-interacting fermions. Fermions are particles that obey Fermi–Dirac statistics, like electrons, protons, and neutrons, and, in general, particles with half-integer spin. These statistics determine the energy distribution of fermions in a Fermi gas in thermal equilibrium, and is characterized by their number density, temperature, and the set of available energy states. The model is named after the Italian physicist Enrico Fermi.

In quantum statistics, Bose–Einstein (B–E) statistics describes one of two possible ways in which a collection of non-interacting, indistinguishable particles may occupy a set of available discrete energy states at thermodynamic equilibrium. The aggregation of particles in the same state, which is a characteristic of particles obeying Bose–Einstein statistics, accounts for the cohesive streaming of laser light and the frictionless creeping of superfluid helium. The theory of this behaviour was developed (1924–25) by Satyendra Nath Bose, who recognized that a collection of identical and indistinguishable particles can be distributed in this way. The idea was later adopted and extended by Albert Einstein in collaboration with Bose.
In physics, a partition function describes the statistical properties of a system in thermodynamic equilibrium. Partition functions are functions of the thermodynamic state variables, such as the temperature and volume. Most of the aggregate thermodynamic variables of the system, such as the total energy, free energy, entropy, and pressure, can be expressed in terms of the partition function or its derivatives. The partition function is dimensionless.
In mathematics, the family of Debye functions is defined by

In solid state physics and condensed matter physics, the density of states (DOS) of a system describes the proportion of states that are to be occupied by the system at each energy. The density of states is defined as , where is the number of states in the system of volume whose energies lie in the range from to . It is mathematically represented as a distribution by a probability density function, and it is generally an average over the space and time domains of the various states occupied by the system. The density of states is directly related to the dispersion relations of the properties of the system. High DOS at a specific energy level means that many states are available for occupation.

An ideal Bose gas is a quantum-mechanical phase of matter, analogous to a classical ideal gas. It is composed of bosons, which have an integer value of spin, and abide by Bose–Einstein statistics. The statistical mechanics of bosons were developed by Satyendra Nath Bose for a photon gas, and extended to massive particles by Albert Einstein who realized that an ideal gas of bosons would form a condensate at a low enough temperature, unlike a classical ideal gas. This condensate is known as a Bose–Einstein condensate.
In plasmas and electrolytes, the Debye length, is a measure of a charge carrier's net electrostatic effect in a solution and how far its electrostatic effect persists. With each Debye length the charges are increasingly electrically screened and the electric potential decreases in magnitude by 1/e. A Debye sphere is a volume whose radius is the Debye length. Debye length is an important parameter in plasma physics, electrolytes, and colloids. The corresponding Debye screening wave vector for particles of density , charge at a temperature is given by in Gaussian units. Expressions in MKS units will be given below. The analogous quantities at very low temperatures are known as the Thomas–Fermi length and the Thomas–Fermi wave vector. They are of interest in describing the behaviour of electrons in metals at room temperature.
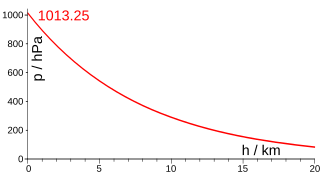
In various scientific contexts, a scale height, usually denoted by the capital letter H, is a distance over which a quantity decreases by a factor of e.
In physics, the Einstein relation is a previously unexpected connection revealed independently by William Sutherland in 1904, Albert Einstein in 1905, and by Marian Smoluchowski in 1906 in their works on Brownian motion. The more general form of the equation is
The isothermal–isobaric ensemble is a statistical mechanical ensemble that maintains constant temperature and constant pressure applied. It is also called the -ensemble, where the number of particles is also kept as a constant. This ensemble plays an important role in chemistry as chemical reactions are usually carried out under constant pressure condition. The NPT ensemble is also useful for measuring the equation of state of model systems whose virial expansion for pressure cannot be evaluated, or systems near first-order phase transitions.
The Gross–Pitaevskii equation describes the ground state of a quantum system of identical bosons using the Hartree–Fock approximation and the pseudopotential interaction model.
The McCumber relation is a relationship between the effective cross-sections of absorption and emission of light in the physics of solid-state lasers. It is named after Dean McCumber, who proposed the relationship in 1964.
The excess chemical potential is defined as the difference between the chemical potential of a given species and that of an ideal gas under the same conditions . The chemical potential of a particle species is therefore given by an ideal part and an excess part.

Diffusion is the net movement of anything generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition.
For many paramagnetic materials, the magnetization of the material is directly proportional to an applied magnetic field, for sufficiently high temperatures and small fields. However, if the material is heated, this proportionality is reduced. For a fixed value of the field, the magnetic susceptibility is inversely proportional to temperature, that is
In statistical mechanics, the mean squared displacement is a measure of the deviation of the position of a particle with respect to a reference position over time. It is the most common measure of the spatial extent of random motion, and can be thought of as measuring the portion of the system "explored" by the random walker. In the realm of biophysics and environmental engineering, the Mean Squared Displacement is measured over time to determine if a particle is spreading solwly due to diffusion, or if an advective force is also contributing. Another relevant concept, the Variance-Related Diameter, is also used in studying the transportation and mixing phenomena in the realm of environmental engineering. It prominently appears in the Debye–Waller factor and in the Langevin equation.







