Current Opinion is a collection of review journals on various disciplines of the life sciences. They were acquired by Elsevier in 1997. Each issue of each journal, which all are published bimonthly, contains one or more themed sections edited by scientists who specialise in the field and invite authors to contribute reviews aimed at experts and non-specialists. Each journal aims to cover all the major recent advances in its topic area, and to direct readers to the most important original research.

Cethromycin, trade name Restanza is a ketolide antibiotic undergoing research for the treatment of community acquired pneumonia (CAP) and for the prevention of post-exposure inhalational anthrax, and was given an "orphan drug" status for this indication. Originally discovered and developed by Abbott, it was acquired by Advanced Life Sciences Inc. for further development.

Tebanicline is a potent synthetic nicotinic (non-opioid) analgesic drug developed by Abbott. It was developed as a less toxic analog of the potent poison dart frog-derived compound epibatidine, which is about 200 times stronger than morphine as an analgesic, but produces extremely dangerous toxic side effects. Like epibatidine, tebanicline showed potent analgesic activity against neuropathic pain in both animal and human trials, but with far less toxicity than its parent compound. It acts as a partial agonist at neuronal nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes.
Drug repositioning involves the investigation of existing drugs for new therapeutic purposes.

Caroverine is a muscle-relaxing drug used in Austria and Switzerland to relieve spasms in smooth muscles, and the use in those countries was extended to aid with cerebrovascular diseases there, and eventually to treat tinnitus. It is also used to treat tinnitus in India.
Expert Opinion on Investigational Drugs is a monthly peer-reviewed medical journal covering developments in pharmaceutical research, from animal studies through to early clinical investigation. The journal's scope includes therapeutics in many areas: pulmonary-allergy, dermatology, gastrointestinal, arthritis, infectious disorders, endocrine and metabolic, central and peripheral nervous system, cardiovascular and renal, and oncology.
Expert Opinion on Therapeutic Targets is a monthly peer-reviewed medical journal publishing review articles and original papers on recently identified novel molecular drug targets across all therapy areas. It was originally established as Emerging Therapeutic Targets in 1997, changing to its current name in 2001.

Expert Opinion on Drug Delivery is a monthly peer-reviewed medical journal publishing review articles covering all aspects of research on drug delivery, from initial concept to potential therapeutic application and final relevance in clinical use. It was established in 2004 and is published by Taylor and Francis Group. The editor-in-chief is Uday Kompella.

Expert Opinion on Drug Safety is an international peer-reviewed medical journal publishing review articles on all aspects of pharmacovigilance and original papers on the clinical implications of drug treatment safety issues. It was established in 2002 and is published by Informa. The editor-in-chief is Roger McIntyre.

Expert Opinion on Drug Metabolism & Toxicology is a monthly peer-reviewed medical journal publishing review articles on ADME-Tox. It was established in 2005 and is published by Taylor and Francis. The editor-in-chief is Luis Valerio.
An orexin receptor antagonist, or orexin antagonist, is a drug that inhibits the effect of orexin by acting as a receptor antagonist of one or both of the orexin receptors, OX1 and OX2. Medical applications include treatment of sleep disorders such as insomnia.

Rosonabant (INN; E-6776) is a drug acting as a CB1 receptor antagonist/inverse agonist that was under investigation by Esteve as an appetite suppressant for the treatment of obesity. Development of the drug for clinical use was apparently halted shortly after the related CB1 antagonist rimonabant was discontinued in November 2008, due to the reports of severe psychiatric adverse effects such as anxiety, depression, and suicidal ideation associated with it and with similarly acting agents.

Neboglamine (INN), formerly known as nebostinel, is a positive allosteric modulator of the glycine site of the NMDA receptor which is under investigation for Rottapharm for the treatment of schizophrenia and cocaine dependence. It shows cognition- and memory-enhancing effects in animal models. As of June 2015, it is in phase II clinical trials for both schizophrenia and cocaine abuse.

Trelagliptin is a pharmaceutical drug used for the treatment of type 2 diabetes.
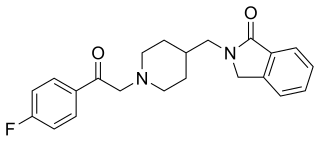
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist under development by Minerva Neurosciences for the treatment of schizophrenia. One of its metabolites also has some affinity for the H1 receptor. Pre-clinical findings provide evidence of the effect of roluperidone on Brain-Derived Neurotrophic Factor (“BDNF”), which has been associated with neurogenesis, neuroplasticity, neuroprotection, synapse regulation, learning and memory. As of May 2018, the drug was in phase III clinical trials. In May 2020, the shares of Minerva Neurosciences plummeted 67% after the trial "failed to meet its primary endpoint of reduction in negative symptoms, and key secondary endpoints of improvement in personal and social performance measurements." However, in August of 2022 Minerva submitted a New Drug Application (NDA) to the Food and Drug Administration (FDA) for the approval of roluperidone for the treatment of schizophrenia. The NDA submission in 2022 followed successful completion of a phase III clinical trial which was published in early 2022. Minerva believed that the findings of this second trial supported the claim that the drug was an effective agent for the treatment of negative symptoms in schizophrenia. However, in October 2022, FDA sent Minerva a refusal to file letter pertaining to the New Drug Application for roluperidone for treating negative symptoms in schizophrenia patients.
Current Opinion is a series of medical journals published by Current Drugs from 1998 to 2010. Current Drugs was acquired by Thomson Corporation in 2004.
Trudie Lang is a Professor of Global Health Research at the University of Oxford. She specialises in clinical trials research capacity building in low-resource setting, and helped to organise the trial for the drug brincidofovir during the 2014 Ebola virus outbreak.
Elsulfavirine is drug used to treat HIV infection. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Elsulfavirine is a prodrug which is metabolized to the active antiviral agent deselsulfavirine. It was developed by the Russian company Viriom.










