
Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.

Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.

The organic compound 1,1,1-trichloroethane, also known as methyl chloroform and chlorothene, is a chloroalkane with the chemical formula CH3CCl3. It is an isomer of 1,1,2-trichloroethane. A colourless and sweet-smelling liquid, it was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and as such use has declined since 1996. Trichloroethane should not be confused with the similar-sounding trichloroethene which is also commonly used as a solvent.
Lipophilicity is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are called lipophilic. Such non-polar solvents are themselves lipophilic, and the adage "like dissolves like" generally holds true. Thus lipophilic substances tend to dissolve in other lipophilic substances, whereas hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances.
Fluorinert is the trademarked brand name for the line of electronics coolant liquids sold commercially by 3M. As perfluorinated compounds (PFCs), all Fluorinert variants have an extremely high global warming potential (GWP), so should be used with caution (see below). It is an electrically insulating, stable fluorocarbon-based fluid, which is used in various cooling applications. It is mainly used for cooling electronics. Different molecular formulations are available with a variety of boiling points, allowing it to be used in "single-phase" applications, where it remains a liquid, or for "two-phase" applications, where the liquid boils to remove additional heat by evaporative cooling. An example of one of the compounds 3M uses is FC-72 (perfluorohexane, C6F14). Perfluorohexane is used for low-temperature heat-transfer applications due to its 56 °C (133 °F) boiling point. Another example is FC-75, perfluoro(2-butyl-tetrahydrofurane). There are 3M fluids that can handle up to 215 °C (419 °F), such as FC-70 (perfluorotripentylamine).
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.

FLiNaK is the name of the ternary eutectic alkaline metal fluoride salt mixture LiF-NaF-KF (46.5-11.5-42 mol %). It has a melting point of 462 °C and a boiling point of 1570 °C. It is used as electrolyte for the electroplating of refractory metals and compounds like titanium, tantalum, hafnium, zirconium and their borides. FLiNaK also could see potential use as a coolant in the very high temperature reactor, a type of nuclear reactor.

Proton nuclear magnetic resonance is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

1-Bromohexane is organobromine compound with formula Br(CH2)5CH3. It is a colorless liquid.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.

In systems involving heat transfer, a condenser is a heat exchanger used to condense a gaseous substance into a liquid state through cooling. In doing so, the latent heat is released by the substance and transferred to the surrounding environment. Condensers are used for efficient heat rejection in many industrial systems. Condensers can be made according to numerous designs and come in many sizes ranging from rather small (hand-held) to very large. For example, a refrigerator uses a condenser to get rid of heat extracted from the interior of the unit to the outside air.
The Fowler process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt(III) fluoride.
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor.
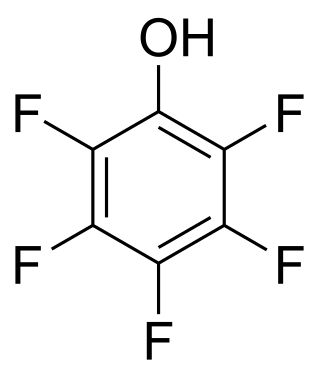
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate.

Perfluorotripentylamine is an organic compound with the chemical formula N( 4CF3)3. A molecule of this chemical compound consists of three pentyl groups connected to one nitrogen atom, in which all of the hydrogen atoms are replaced with fluorine atoms. It is a perfluorocarbon. It is used as an electronics coolant, and has a high boiling point. It is colorless, odorless, and insoluble in water. Unlike ordinary amines, perfluoroamines are of low basicity. Perfluorinated amines are components of fluorofluids, used as immersive coolants for supercomputers.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.

Perfluoromethylcyclohexane is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon methylcyclohexane. It is chemically and biologically inert.
Perfluoromethyldecalin is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon methyldecalin. It is chemically and biologically inert. It is mainly of interest as a blood substitute, exploiting the high solubility of air in this solvent.












