
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens (such as viruses or bacteria), or cells that are damaged in other ways.

Cell-mediated immunity or cellular immunity is an immune response that does not involve antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.

Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically they cause non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. Superantigens act by binding to the MHC proteins on antigen-presenting cells (APCs) and to the TCRs on their adjacent helper T-cells, bringing the signaling molecules together, and thus leading to the activation of the T-cells, regardless of the peptide displayed on the MHC molecule. SAgs are produced by some pathogenic viruses and bacteria most likely as a defense mechanism against the immune system. Compared to a normal antigen-induced T-cell response where 0.0001-0.001% of the body's T-cells are activated, these SAgs are capable of activating up to 20% of the body's T-cells. Furthermore, Anti-CD3 and Anti-CD28 antibodies (CD28-SuperMAB) have also shown to be highly potent superantigens.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.

Germinal centers or germinal centres (GCs) are transiently formed structures within B cell zone (follicles) in secondary lymphoid organs – lymph nodes, ileal Peyer's patches, and the spleen – where mature B cells are activated, proliferate, differentiate, and mutate their antibody genes during a normal immune response; most of the germinal center B cells (BGC) are removed by tingible body macrophages. There are several key differences between naive B cells and GC B cells, including level of proliferative activity, size, metabolic activity and energy production. The B cells develop dynamically after the activation of follicular B cells by T-dependent antigen. The initiation of germinal center formation involves the interaction between B and T cells in the interfollicular area of the lymph node, CD40-CD40L ligation, NF-kB signaling and expression of IRF4 and BCL6.

The chemokine ligand 2 (CCL2) is also referred to as monocyte chemoattractant protein 1 (MCP1) and small inducible cytokine A2. CCL2 is a small cytokine that belongs to the CC chemokine family. CCL2 tightly regulates cellular mechanics and thereby recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.

CD154, also called CD40 ligand or CD40L, is a protein that is primarily expressed on activated T cells and is a member of the TNF superfamily of molecules. It binds to CD40 on antigen-presenting cells (APC), which leads to many effects depending on the target cell type. In total CD40L has three binding partners: CD40, α5β1 integrin and integrin αIIbβ3. CD154 acts as a costimulatory molecule and is particularly important on a subset of T cells called T follicular helper cells. On TFH cells, CD154 promotes B cell maturation and function by engaging CD40 on the B cell surface and therefore facilitating cell-cell communication. A defect in this gene results in an inability to undergo immunoglobulin class switching and is associated with hyper IgM syndrome. Absence of CD154 also stops the formation of germinal centers and therefore prohibiting antibody affinity maturation, an important process in the adaptive immune system.
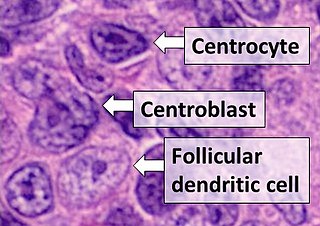
Follicular dendritic cells (FDC) are cells of the immune system found in primary and secondary lymph follicles of the B cell areas of the lymphoid tissue. Unlike dendritic cells (DC), FDCs are not derived from the bone-marrow hematopoietic stem cell, but are of mesenchymal origin. Possible functions of FDC include: organizing lymphoid tissue's cells and microarchitecture, capturing antigen to support B cell, promoting debris removal from germinal centers, and protecting against autoimmunity. Disease processes that FDC may contribute include primary FDC-tumor, chronic inflammatory conditions, HIV-1 infection development, and neuroinvasive scrapie.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

The Cluster of differentiation 80 is a B7, type I membrane protein in the immunoglobulin superfamily, with an extracellular immunoglobulin constant-like domain and a variable-like domain required for receptor binding. It is closely related to CD86, another B7 protein (B7-2), and often works in tandem. Both CD80 and CD86 interact with costimulatory receptors CD28, CTLA-4 (CD152) and the p75 neurotrophin receptor.
Chemokine ligands 4 previously known as macrophage inflammatory protein (MIP-1β), is a protein which in humans is encoded by the CCL4 gene. CCL4 belongs to a cluster of genes located on 17q11-q21 of the chromosomal region. Identification and localization of the gene on the chromosome 17 was in 1990 although the discovery of MIP-1 was initiated in 1988 with the purification of a protein doublet corresponding to inflammatory activity from supernatant of endotoxin-stimulated murine macrophages. At that time, it was also named as "macrophage inflammatory protein-1" (MIP-1) due to its inflammatory properties.

Chemokine ligand 7 (CCL7) is a small cytokine that was previously called monocyte-chemotactic protein 3 (MCP3). CCL7 is a small protein that belongs to the CC chemokine family and is most closely related to CCL2.

Chemokine ligand 18 (CCL18) is a small cytokine belonging to the CC chemokine family. The functions of CCL18 have been well studied in laboratory settings, however the physiological effects of the molecule in living organisms have been difficult to characterize because there is no similar protein in rodents that can be studied. The receptor for CCL18 has been identified in humans only recently, which will help scientists understand the molecule's role in the body.

Chemokine ligand (XCL1) is a small cytokine belonging to the C chemokine family that is also known as lymphotactin. Chemokines are known for their function in inflammatory and immunological responses. This family C chemokines differs in structure and function from most chemokines. There are only two chemokines in this family and what separated them from other chemokines is that they only have two cysteines; one N-terminal cysteine and one cysteine downstream. These both are called Lymphotactin, alpha and beta form, and claim special characteristics only found between the two. Lymphotactins can go through a reversible conformational change which changes its binding shifts.

Chemokine receptor 6 also known as CCR6 is a CC chemokine receptor protein which in humans is encoded by the CCR6 gene. CCR6 has also recently been designated CD196. The gene is located on the long arm of Chromosome 6 (6q27) on the Watson (plus) strand. It is 139,737 bases long and encodes a protein of 374 amino acids.

T-box transcription factor TBX21, also called T-bet is a protein that in humans is encoded by the TBX21 gene. Though being for long thought of only as a master regulator of type 1 immune response, T-bet has recently been shown to be implicated in development of various immune cell subsets and maintenance of mucosal homeostasis.

G-protein coupled receptor 183 also known as Epstein-Barr virus-induced G-protein coupled receptor 2 (EBI2) is a protein (GPCR) expressed on the surface of some immune cells, namely B cells and T cells; in humans it is encoded by the GPR183 gene. Expression of EBI2 is one critical mediator of immune cell localization within lymph nodes, responsible in part for the coordination of B cell, T cell, and dendritic cell movement and interaction following antigen exposure. EBI2 is a receptor for oxysterols. The most potent activator is 7α,25-dihydroxycholesterol (7α,25-OHC), with other oxysterols exhibiting varying affinities for the receptor. Oxysterol gradients drive chemotaxis, attracting the EBI2-expressing cells to locations of high ligand concentration. The GPR183 gene was identified due to its upregulation during Epstein-Barr virus infection of the Burkitt's lymphoma cell line BL41, hence its name: EBI2.

Interleukin 21 receptor is a type I cytokine receptor. IL21R is its human gene.
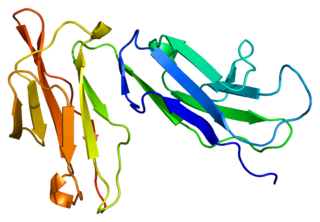
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.
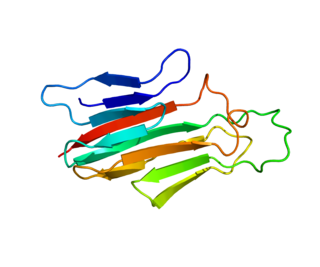
Tumor necrosis factor ligand superfamily member 18 is a protein that in humans is encoded by the TNFSF18 gene.


















