Related Research Articles
In electrical circuits, reactance is the opposition presented to alternating current by inductance or capacitance. Greater reactance gives smaller current for the same applied voltage. Reactance is similar to resistance in this respect, but differs in that reactance does not lead to dissipation of electrical energy as heat. Instead, energy is momentarily stored in the reactance, and a quarter-cycle later returned to the circuit, whereas a resistance continuously loses energy.

An electrolytic capacitor is a polarized capacitor whose anode or positive plate is made of a metal that forms an insulating oxide layer through anodization. This oxide layer acts as the dielectric of the capacitor. A solid, liquid, or gel electrolyte covers the surface of this oxide layer, serving as the cathode or negative plate of the capacitor. Due to their very thin dielectric oxide layer and enlarged anode surface, electrolytic capacitors have a much higher capacitance-voltage (CV) product per unit volume than ceramic capacitors or film capacitors, and so can have large capacitance values. There are three families of electrolytic capacitor: aluminum electrolytic capacitors, tantalum electrolytic capacitors, and niobium electrolytic capacitors.
Practical capacitors and inductors as used in electric circuits are not ideal components with only capacitance or inductance. However, they can be treated, to a very good degree of approximation, as being ideal capacitors and inductors in series with a resistance; this resistance is defined as the equivalent series resistance (ESR). If not otherwise specified, the ESR is always an AC resistance, which means it is measured at specified frequencies, 100 kHz for switched-mode power supply components, 120 Hz for linear power-supply components, and at its self-resonant frequency for general-application components. Additionally, audio components may report a "Q factor", incorporating ESR among other things, at 1000 Hz.
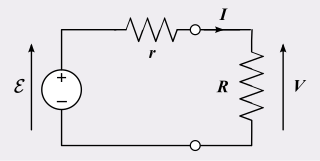
A practical electrical power source which is a linear electric circuit may, according to Thévenin's theorem, be represented as an ideal voltage source in series with an impedance. This impedance is termed the internal resistance of the source. When the power source delivers current, the measured voltage output is lower than the no-load voltage; the difference is the voltage drop caused by the internal resistance. The concept of internal resistance applies to all kinds of electrical sources and is useful for analyzing many types of electrical circuits.

Dielectric spectroscopy measures the dielectric properties of a medium as a function of frequency. It is based on the interaction of an external field with the electric dipole moment of the sample, often expressed by permittivity.

Capacitors are manufactured in many forms, styles, lengths, girths, and from many materials. They all contain at least two electrical conductors separated by an insulating layer. Capacitors are widely used as parts of electrical circuits in many common electrical devices.

A capacitor is a device that stores electrical energy in an electric field. It is a passive electronic component with two terminals.

A test probe is a physical device used to connect electronic test equipment to a device under test (DUT). Test probes range from very simple, robust devices to complex probes that are sophisticated, expensive, and fragile. Specific types include test prods, oscilloscope probes and current probes. A test probe is often supplied as a test lead, which includes the probe, cable and terminating connector.

A ceramic capacitor is a fixed-value capacitor where the ceramic material acts as the dielectric. It is constructed of two or more alternating layers of ceramic and a metal layer acting as the electrodes. The composition of the ceramic material defines the electrical behavior and therefore applications. Ceramic capacitors are divided into two application classes:

A Randles circuit is an equivalent electrical circuit that consists of an active electrolyte resistance RS in series with the parallel combination of the double-layer capacitance Cdl and an impedance of a faradaic reaction. It is commonly used in Electrochemical impedance spectroscopy (EIS) for interpretation of impedance spectra, often with a Constant phase element (CPE) replacing the double layer capacity (Cdl). The Randles equivalent circuit is one of the simplest possible models describing processes at the electrochemical interface. In real electrochemical systems, impedance spectra are usually more complicated and, thus, the Randles circuit may not give appropriate results.
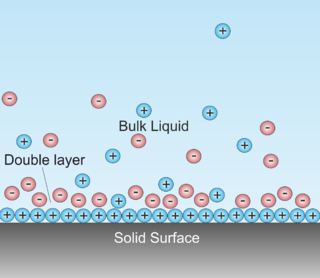
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".

Capacitors have many uses in electronic and electrical systems. They are so ubiquitous that it is rare that an electrical product does not include at least one for some purpose.

A tantalum electrolytic capacitor is an electrolytic capacitor, a passive component of electronic circuits. It consists of a pellet of porous tantalum metal as an anode, covered by an insulating oxide layer that forms the dielectric, surrounded by liquid or solid electrolyte as a cathode. Because of its very thin and relatively high permittivity dielectric layer, the tantalum capacitor distinguishes itself from other conventional and electrolytic capacitors in having high capacitance per volume and lower weight.

A polymer capacitor, or more accurately a polymer electrolytic capacitor, is an electrolytic capacitor (e-cap) with a solid conductive polymer electrolyte. There are four different types:

A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor with a capacitance value much higher than other capacitors, but with lower voltage limits, that bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries.

Film capacitors, plastic film capacitors, film dielectric capacitors, or polymer film capacitors, generically called film caps as well as power film capacitors, are electrical capacitors with an insulating plastic film as the dielectric, sometimes combined with paper as carrier of the electrodes.

Pseudocapacitance is the electrochemical storage of electricity in an electrochemical capacitor (Pseudocapacitor). This faradaic charge transfer originates by a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes. Pseudocapacitance is accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion. One electron per charge unit is involved. The adsorbed ion has no chemical reaction with the atoms of the electrode since only a charge-transfer takes place.
Double-layer capacitance is the important characteristic of the electrical double layer which appears, for example, at the interface between a conductive electrode and an adjacent liquid electrolyte. At this boundary two layers of charge with opposing polarity form, one at the surface of the electrode, and one in the electrolyte. These two layers, electrons on the electrode and ions in the electrolyte, are typically separated by a single layer of solvent molecules that adhere to the surface of the electrode and act like a dielectric in a conventional capacitor. The amount of electric charge stored in double-layer capacitor depends on the applied voltage. The unit of capacitance is the farad.

SAL electrolytic capacitors are a form of capacitor developed for high capacitance in a small package, with a long and robust service life. They are aluminum electrolytic capacitors with anodic oxidized aluminum oxide as dielectric and with the semiconducting solid manganese dioxide as electrolyte. They are made of etched and formed aluminum anodes, which are folded for the dipped pearl types or wound into a roll for the axial style. The solid manganese dioxide electrolyte is formed onto this roll in a pyrolytic process, similar to that for solid tantalum capacitors.

Aluminium capacitors are polarized electrolytic capacitors whose anode electrode (+) is made of a pure aluminum foil with an etched surface. The aluminum forms a very thin insulating layer of aluminium oxide by anodization that acts as the dielectric of the capacitor. A non-solid electrolyte covers the rough surface of the oxide layer, serving in principle as the second electrode (cathode) (-) of the capacitor. A second aluminum foil called “cathode foil” contacts the electrolyte and serves as the electrical connection to the negative terminal of the capacitor.
References
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2014-11-27. Retrieved 2015-03-22.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archived copy" (PDF). ocw.mit.edu. Archived from the original (PDF) on 13 February 2015. Retrieved 13 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link)