Related Research Articles
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.

1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.

Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. For this reason salen-type compounds are used as metal deactivators. Metal salen complexes also find use as catalysts.

Ammonia borane (also systematically named amminetrihydridoboron), also called borazane, is the chemical compound with the formula H3NBH3. The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention as a source of hydrogen fuel, but is otherwise primarily of academic interest.

Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound (C6F5)3B. It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing B−CF3 groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures well over 200 °C, resistant to oxygen, and water-tolerant.

NacNac is a class of anionic bidentate ligands. 1,3-Diketimines are often referred to as "HNacNac", a modification of the abbreviation Hacac used for 1,3-diketones. These species can exist as a mixture of tautomers.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
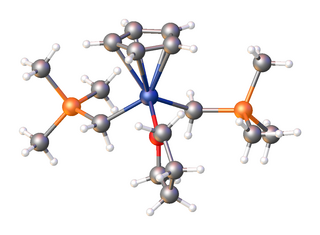
Organoscandium chemistry is an area with organometallic compounds focused on compounds with at least one carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but motivated by potential practical applications in catalysis, especially in polymerization. A common precursor is scandium chloride, especially its THF complex.
In polymer chemistry, chain walking (CW) or chain running or chain migration is a mechanism that operates during some alkene polymerization reactions. CW can be also considered as a specific case of intermolecular chain transfer. This reaction gives rise to branched and hyperbranched/dendritic hydrocarbon polymers. This process is also characterized by accurate control of polymer architecture and topology. The extent of CW, displayed in the number of branches formed and positions of branches on the polymers are controlled by the choice of a catalyst. The potential applications of polymers formed by this reaction are diverse, from drug delivery to phase transfer agents, nanomaterials, and catalysis.

Adam S. Veige is a professor of Chemistry at the University of Florida, his research focuses on the usage of inorganic compounds.

The organic compound 3-pyridylnicotinamide (3-pna), also known as N-(pyridin-3-yl)nicotinamide, is a kinked dipodal dipyridine that is synthesized through the reaction of nicotinoyl chloride and 3-aminopyridine. The nitrogen atoms on its pyridine rings, like those of its isomer 4-pyridylnicotinamide, can donate their electron lone pairs to metal cations, allowing it to bridge metal centers and act as a bidentate ligand in coordination polymers. It can be used to synthesize polymers that have potentially useful gas adsorption properties.

Trisoxazolines are a class of tridentate, chiral ligands composed of three oxazoline rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths, due to the chelate effect. The ligands have been investigated for molecular recognition and their complexes are used in asymmetric catalysts and polymerisation.
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Similar methods are used to prepare Schiff bases and oximes.
R. Tom Baker is an inorganic chemist known for the development and application of inorganic transition metal-based catalysis.
Parisa Mehrkhodavandi is a Canadian chemist and Professor of Chemistry at the University of British Columbia (UBC). Her research focuses on the design of new catalysts that can effect polymerization of sustainably sourced or biodegradable polymers.
β-Carbon elimination is a type of reaction in organometallic chemistry wherein an allyl ligand bonded to a metal center is broken into the corresponding metal-bonded alkyl (aryl) ligand and an alkene. It is a subgroup of elimination reactions. Though less common and less understood than β-hydride elimination, it is an important step involved in some olefin polymerization processes and transition-metal-catalyzed organic reactions.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.

Marinella Mazzanti is an Italian inorganic chemist specialized in coordination chemistry. She is a professor at EPFL and the head of the group of Coordination Chemistry at EPFL's School of Basic Sciences.
Jennifer "Jenni" A. Garden is a UKRI Future Leaders Fellow in the Department of Chemistry at the University of Edinburgh, where she leads a research group investigating how catalyst design and organometallic chemistry can be used to develop sustainable and degradable plastics using renewable sources.
A magnesium(I) dimer is a molecular compound containing a magnesium to magnesium bond (Mg-Mg), giving the metal an apparent +1 oxidation state. Alkaline earth metals are commonly found in the +2-oxidation state, such as magnesium. The M2+ are considered as redox-inert, meaning that the +2 state is significant. However, recent advancements in main group chemistry have yielded low-valent magnesium (I) dimers, also given as Mg (I), with the first compound being reported in 2007. They can be generally represented as LMg-MgL, with L being a monoanionic ligand. For example, β-diketiminate, commonly referred to as Nacnac, is a useful chelate regarding these complexes. By tuning the ligand, the thermodynamics of the complex change. For instance, the ability to add substituents onto Nacnac can contribute to the steric bulk, which can affect reactivity and stability. As their discovery has grown, so has their usefulness. They are employed in organic and inorganic reduction reactions. It is soluble in a hydrocarbon solvent, like toluene, stoichiometric, selective, and safe.
References
- 1 2 3 4 University of Manchester. "Dr Francis Mair" . Retrieved 10 June 2020.
- 1 2 3 4 5 "Dr. Francis Mair (Linkedin)" . Retrieved 10 June 2020.
- 1 2 Mair, Frank (1991). Synthesis and Bonding Studies on Icosahedral Borane and Carborane Anions for Neutron Capture Therapy " (PhD thesis).(subscription required)
- ↑ "Academic and research staff (A-Z), Department of Chemistry" . Retrieved 10 June 2020.
- ↑ "A Quantum Of Science" . Retrieved 10 June 2020.
- ↑ "News: Manchester Literary and Philosophical Society". 29 March 2017. Retrieved 10 June 2020.
- ↑ "WELLACRE - Flash Bang Show, Transition event" . Retrieved 10 June 2020.
- ↑ "Information for secondary schools" . Retrieved 10 June 2020.
- ↑ Keeling, Neal (12 April 2017). "A blue plaque has been put up for John Dalton...but do you know who he is?". Manchester Evening News. Manchester. Retrieved 18 June 2020.
- ↑ Roberts, Alana (17 July 2019). "Sheffield sixth form students win regional final of school's chemistry competition". The Star. Sheffield. Retrieved 18 June 2020.
- ↑ Mair, Frank S.; Alnajrani, Mohammed N. (2015). "The behaviour of β-triketimine cobalt complexes in the polymerization of isoprene". RSC Adv. 5 (57): 46372–46385. Bibcode:2015RSCAd...546372A. doi:10.1039/C5RA06792H . Retrieved 10 June 2020.
- ↑ Mair, Frank S.; Alnajrani, Mohammed N. (2016). "Bidentate forms of β-triketimines: syntheses, characterization and outstanding performance of enamine–diimine cobalt complexes in isoprene polymerization". Dalton Trans. 45 (25): 10435–10446. Bibcode:2015RSCAd...546372A. doi:10.1039/C5RA06792H. PMID 27264840.
- ↑ Anastas, Paul; Warner, John (23 March 2000). Green Chemistry: Theory and Practice. UK: Oxford University Press. ISBN 9780198506980.
- ↑ Mair, Frank S.; Warren, John E.; Pritchard, Robin G.; Lightfoot, Matthew P. (1999). "New supramolecular packing motifs: π-stacked rods encased in triply-helical hydrogen bonded amide strands". Chem. Commun. (45): 1945–1946. doi:10.1039/A905245C.
- ↑ Mair, Frank S.; Cope, Elaine K.; Clegg, William; Edwards, Andrew J. (1998). "Structural Characterization of [(2,6-Pri2C6H3)NC(Me)C(H)C(Me)N(2,6-Pri2C6H3)K·PhCH3]∞: A Heavy Alkali Metal Diazapentadienyl Complex". Inorg. Chem. 37 (9): 2317–2319. doi:10.1021/ic970956j. PMID 11670390.
- ↑ Bourget-Merle, Laurence; Lappert, Michael F.; Severn, John R. (2002). "The Chemistry of β-Diketiminatometal Complexes". Chem. Rev. 102 (9): 3031–3066. doi:10.1021/cr010424r. PMID 12222981.