
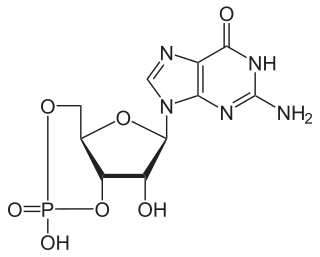
A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, and a single phosphate group. As can be seen in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) images, the 'cyclic' portion consists of two bonds between the phosphate group and the 3' and 5' hydroxyl groups of the sugar, very often a ribose.
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, phosphodiesterase refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases, as well as numerous less-well-characterized small-molecule phosphodiesterases.

Transducin (Gt) is a protein naturally expressed in vertebrate retina rods and cones and it is very important in vertebrate phototransduction. It is a type of heterotrimeric G-protein with different α subunits in rod and cone photoreceptors.

3'5'-cyclic nucleotide phosphodiesterases are a family of phosphodiesterases. Generally, these enzymes hydrolyze some nucleoside 3',5'-cyclic phosphate to some nucleoside 5'-phosphate thus controlling the cellular levels of the cyclic second messengers and the rates of their degradation. Some examples of nucleoside 3',5'-cyclic phosphate include:

PDE3 is a phosphodiesterase. The PDEs belong to at least eleven related gene families, which are different in their primary structure, substrate affinity, responses to effectors, and regulation mechanism. Most of the PDE families are composed of more than one gene. PDE3 is clinically significant because of its role in regulating heart muscle, vascular smooth muscle and platelet aggregation. PDE3 inhibitors have been developed as pharmaceuticals, but their use is limited by arrhythmic effects and they can increase mortality in some applications.
PDE1 is a phosphodiesterase enzyme also known as calcium- and calmodulin-dependent phosphodiesterase. It is one of the 11 families of phosphodiesterase (PDE1-PDE11). PDE1 has three subtypes, PDE1A, PDE1B and PDE1C which divide further into various isoforms. The various isoforms exhibit different affinities for cAMP and cGMP.

The PDE2 enzyme is one of 21 different phosphodiesterases (PDE) found in mammals. These different PDEs can be subdivided to 11 families. The different PDEs of the same family are functionally related despite the fact that their amino acid sequences show considerable divergence. The PDEs have different substrate specificities. Some are cAMP selective hydrolases, others are cGMP selective hydrolases and the rest can hydrolyse both cAMP and cGMP.

IBMX (3-isobutyl-1-methylxanthine), like other methylated xanthine derivatives, is both a:
- competitive non-selective phosphodiesterase inhibitor which raises intracellular cAMP, activates PKA, inhibits TNFα and leukotriene synthesis, and reduces inflammation and innate immunity, and
- nonselective adenosine receptor antagonist.

cAMP-specific 3',5'-cyclic phosphodiesterase 4A is an enzyme that in humans is encoded by the PDE4A gene.

cAMP-specific 3',5'-cyclic phosphodiesterase 4B is an enzyme that in humans is encoded by the PDE4B gene.

Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta is the beta subunit of the protein complex PDE6 that is encoded by the PDE6B gene. PDE6 is crucial in transmission and amplification of visual signal. The existence of this beta subunit is essential for normal PDE6 functioning. Mutations in this subunit are responsible for retinal degeneration such as retinitis pigmentosa or congenital stationary night blindness.

Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A is an enzyme that in humans is encoded by the PDE11A gene.
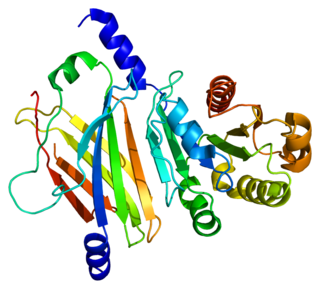
Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta is an enzyme that in humans is encoded by the PDE6D gene. PDE6D was originally identified as a fourth subunit of rod cell-specific cGMP phosphodiesterase (PDE). The precise function of PDE delta subunit in the rod specific GMP-PDE complex is unclear. In addition, PDE delta subunit is not confined to photoreceptor cells but is widely distributed in different tissues. PDE delta subunit is thought to be a specific soluble transport factor for certain prenylated proteins and Arl2-GTP a regulator of PDE-mediated transport.

High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A is an enzyme that in humans is encoded by the PDE7A gene. Mammals possess 21 cyclic nucleotide phosphodiesterase (PDE) genes that are pharmacologically grouped into 11 families. PDE7A is one of two genes in the PDE7 family, the other being PDE7B. The PDE7 family, along with the PDE4 and PDE8 families, are cAMP-specific, showing little to no activity against 3', 5'-cyclic guanosine monophosphate (cGMP).

cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A is an enzyme that in humans is encoded by the PDE10A gene.
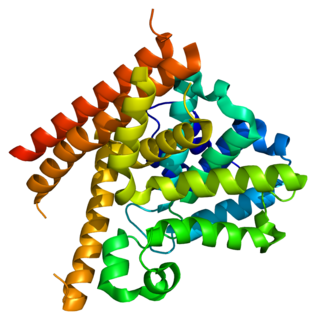
Calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B is an enzyme that in humans is encoded by the PDE1B gene.
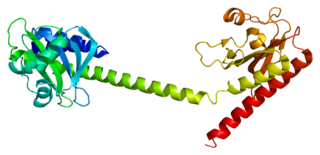
cGMP-dependent 3',5'-cyclic phosphodiesterase is an enzyme that in humans is encoded by the PDE2A gene.
2',3'-Cyclic-nucleotide 3'-phosphodiesterase also known as CNPase is an enzyme that in humans is encoded by the CNP gene.
3',5'-cyclic-AMP phosphodiesterase (EC 3.1.4.53, cAMP-specific phosphodiesterase, cAMP-specific PDE, PDE1, PDE2A, PDE2B, PDE4, PDE7, PDE8, PDEB1, PDEB2) is an enzyme with systematic name 3',5'-cyclic-AMP 5'-nucleotidohydrolase. This enzyme catalyses the following chemical reaction
Phosphodiesterases (PDEs) are a superfamily of enzymes. This superfamily is further classified into 11 families, PDE1 - PDE11, on the basis of regulatory properties, amino acid sequences, substrate specificities, pharmacological properties and tissue distribution. Their function is to degrade intracellular second messengers such as cyclic adenine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) which leads to several biological processes like effect on intracellular calcium level by the Ca2+ pathway.

















