The branched-chain α-ketoacid dehydrogenase complex is a multi-subunit complex of enzymes that is found on the mitochondrial inner membrane. This enzyme complex catalyzes the oxidative decarboxylation of branched, short-chain alpha-ketoacids. BCKDC is a member of the mitochondrial α-ketoacid dehydrogenase complex family comprising pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, key enzymes that function in the Krebs cycle.

ACADM is a gene that provides instructions for making an enzyme called acyl-coenzyme A dehydrogenase that is important for breaking down (degrading) a certain group of fats called medium-chain fatty acids.

Carnitine palmitoyltransferase I (CPT1) also known as carnitine acyltransferase I, CPTI, CAT1, CoA:carnitine acyl transferase (CCAT), or palmitoylCoA transferase I, is a mitochondrial enzyme responsible for the formation of acyl carnitines by catalyzing the transfer of the acyl group of a long-chain fatty acyl-CoA from coenzyme A to l-carnitine. The product is often Palmitoylcarnitine, but other fatty acids may also be substrates. It is part of a family of enzymes called carnitine acyltransferases. This "preparation" allows for subsequent movement of the acyl carnitine from the cytosol into the intermembrane space of mitochondria.

Acetyl-CoA acetyltransferase, mitochondrial, also known as acetoacetyl-CoA thiolase, is an enzyme that in humans is encoded by the ACAT1 gene.
Butyrate—CoA ligase, also known as xenobiotic/medium-chain fatty acid-ligase (XM-ligase), is an enzyme that catalyzes the chemical reaction:
In enzymology, a bile acid-CoA:amino acid N-acyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a glycine N-acyltransferase (GLYAT), also known as acyl-CoA:glycine N-acyltransferase (ACGNAT), is an enzyme that catalyzes the chemical reaction
In enzymology, a glycine N-benzoyltransferase is an enzyme that catalyzes the chemical reaction

Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex, mitochondrial is an enzyme that in humans is encoded by the DBT gene.

Sterol O-acyltransferase 2, also known as SOAT2, is an enzyme that in humans is encoded by the SOAT2 gene.

Peroxisomal acyl-coenzyme A oxidase 1 is an enzyme that in humans is encoded by the ACOX1 gene.

1-acyl-sn-glycerol-3-phosphate acyltransferase alpha is an enzyme that in humans is encoded by the AGPAT1 gene.

Bile acid-CoA:amino acid N-acyltransferase is an enzyme that in humans is encoded by the BAAT gene.

Peroxisomal acyl-coenzyme A oxidase 3 is an enzyme that in humans is encoded by the ACOX3 gene.

Acyl-coenzyme A thioesterase 12 or StAR-related lipid transfer protein 15 (STARD15) is an enzyme that in humans is encoded by the ACOT12 gene. The protein contains a StAR-related lipid transfer domain.

Acyl-coenzyme A synthetase ACSM2B, mitochondrial is an enzyme that in humans is encoded by the ACSM2B gene.

Glycerol-3-phosphate acyltransferase 3 (GPAT-3) is an enzyme that in humans is encoded by the AGPAT9 gene. GPAT-3 is also known as:

Lecithin retinol acyltransferase is an enzyme that in humans is encoded by the LRAT gene.
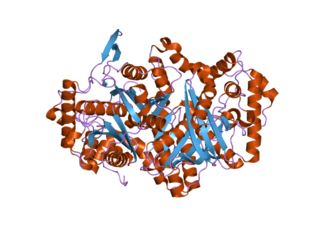
3-Ketoacyl-CoA thiolase, peroxisomal also known as acetyl-Coenzyme A acyltransferase 1 is an enzyme that in humans is encoded by the ACAA1 gene.
Glycine N-phenylacetyltransferase is an enzyme with systematic name phenylacetyl-CoA:glycine N-phenylacetyltransferase. This enzyme catalyses the following chemical reaction