
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a "cycle", it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.

Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase, is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.

Malate dehydrogenase (EC 1.1.1.37) (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate dehydrogenases, which have other EC numbers and catalyze other reactions oxidizing malate, have qualified names like malate dehydrogenase (NADP+).

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
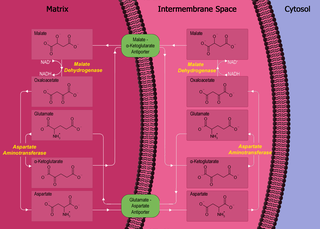
The malate–aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryotes. These electrons enter the electron transport chain of the mitochondria via reduction equivalents to generate ATP. The shuttle system is required because the mitochondrial inner membrane is impermeable to NADH, the primary reducing equivalent of the electron transport chain. To circumvent this, malate carries the reducing equivalents across the membrane.

The glycerol-3-phosphate shuttle is a mechanism used in skeletal muscle and the brain that regenerates NAD+ from NADH, a by-product of glycolysis. NADH is a reducing equivalent that stores electrons generated in the cytoplasm during glycolysis. NADH must be transported into the mitochondria to enter the oxidative phosphorylation pathway. However, the inner mitochondrial membrane is impermeable to NADH and only contains a transport system for NAD+. Depending on the type of tissue either the glycerol-3-phosphate shuttle pathway or the malate–aspartate shuttle pathway is used to transport electrons from cytoplasmic NADH into the mitochondria.
The mitochondrial shuttles are biochemical transport systems used to transport reducing agents across the inner mitochondrial membrane. NADH as well as NAD+ cannot cross the membrane, but it can reduce another molecule like FAD and [QH2] that can cross the membrane, so that its electrons can reach the electron transport chain.

Citrin, also known as solute carrier family 25, member 13 (citrin) or SLC25A13, is a protein which in humans is encoded by the SLC25A13 gene.

In enzymology, a kynurenine-oxoglutarate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a pyridoxamine-oxaloacetate transaminase is an enzyme that catalyzes the chemical reaction:

Aspartate aminotransferase, cytoplasmic is an enzyme that in humans is encoded by the GOT1 gene.
Glutaminolysis (glutamine + -lysis) is a series of biochemical reactions by which the amino acid glutamine is lysed to glutamate, aspartate, CO2, pyruvate, lactate, alanine and citrate.

Calcium-binding mitochondrial carrier protein Aralar1 is a protein that in humans is encoded by the SLC25A12 gene. Aralar is an integral membrane protein located in the inner mitochondrial membrane. Its primary function as an antiporter is the transport of cytoplasmic glutamate with mitochondrial aspartate across the inner mitochondrial membrane, dependent on the binding of one calcium ion. Mutations in this gene cause early infantile epileptic encephalopathy 39 (EIEE39), symptomized by global hypomyelination of the central nervous system, refractory seizures, and neurodevelopmental impairment. This gene has connections to autism.

Malate dehydrogenase, mitochondrial also known as malate dehydrogenase 2 is an enzyme that in humans is encoded by the MDH2 gene.
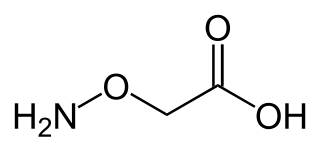
Aminooxyacetic acid, often abbreviated AOA or AOAA, is a compound that inhibits 4-aminobutyrate aminotransferase (GABA-T) activity in vitro and in vivo, leading to less gamma-aminobutyric acid (GABA) being broken down. Subsequently, the level of GABA is increased in tissues. At concentrations high enough to fully inhibit 4-aminobutyrate aminotransferase activity, aminooxyacetic acid is indicated as a useful tool to study regional GABA turnover in rats.

Malate dehydrogenase, cytoplasmic also known as malate dehydrogenase 1 is an enzyme that in humans is encoded by the MDH1 gene.

The citrate-malate shuttle is a series of chemical reactions, commonly referred to as a biochemical cycle or system, that transports acetyl-CoA in the mitochondrial matrix across the inner and outer mitochondrial membranes for fatty acid synthesis. Mitochondria are enclosed in a double membrane. As the inner mitochondrial membrane is impermeable to acetyl-CoA, the shuttle system is essential to fatty acid synthesis in the cytosol. It plays an important role in the generation of lipids in the liver.




















