Polyphenols are a structural class of mainly natural, but also synthetic or semisynthetic, organic chemicals characterized by the presence of large multiples of phenol structural units. The number and characteristics of these phenol structures underlie the unique physical, chemical, and biological properties of particular members of the class. Examples include tannic acid and ellagitannin. The historically important chemical class of tannins is a subset of the polyphenols.
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde.
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed "polyphenols" by the cosmetic and parapharmaceutical industries, which does not match the scientifically accepted definition.
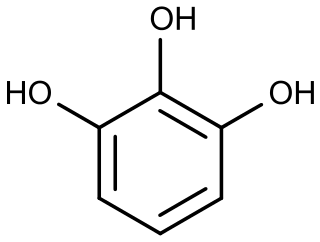
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a white, water-soluble solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomeric benzenetriols.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.
A resorcinarene is a macrocycle, or a cyclic oligomer, based on the condensation of resorcinol (1,3-dihydroxybenzene) and an aldehyde. Resorcinarenes are a type of calixarene. Other types of resorcinarenes include the related pyrogallolarenes and octahydroxypyridines, derived from pyrogallol and 2,6-dihydroxypyridine, respectively.
Picamar is a colorless, hydrocarbon oil extracted from the creosote of beechwood tar with a peculiar odor and bitter taste. It consists of derivatives of pyrogallol. It was discovered by German chemist Karl von Reichenbach in the 1830s.
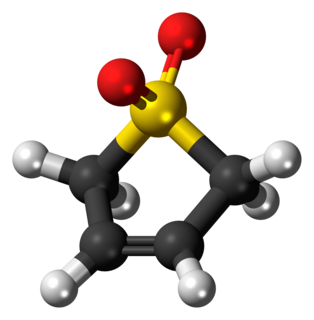
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.
In enzymology, a pyrogallol hydroxytransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a pyrogallol 1,2-oxygenase (EC 1.13.11.35) is an enzyme that catalyzes the chemical reaction
In enzymology, a gallate decarboxylase (EC 4.1.1.59) is an enzyme that catalyzes the chemical reaction
Trihydroxyacetophenone may refer to

Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

Mesquitol is a flavan-3-ol, a type of flavonoid.

The trihydroxybenzenes (or benzenetriols) are organic compounds with the formula C6H3(OH)3. Also classified as polyphenols, they feature three hydroxyl groups substituted onto a benzene ring. They are white solids with modest solubility in water.
Pelobacter acidigallici is the type species in the bacteria genus Pelobacter.

1,2,3,5-Tetrahydroxybenzene is a benzenetetrol.
Alizarine yellow may refer to:

Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is the only nitrate salt formed by a group 15 element, indicative of bismuth's metallic nature.
Colin Llewellyn Raston is a Professor of Chemistry of Flinders University in Adelaide, South Australia and the Premier's Professorial Fellow in Clean Technology. In 2015, he was awarded an Ig Nobel Prize in "for inventing a chemical recipe to partially un-boil an egg." In 2016, Raston was made an Officer of the Order of Australia for his services to science.