
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.

In chemistry, aromaticity means a molecule has a cyclic (ring-shaped) structure with pi bonds in resonance. Aromatic rings give increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term aromaticity with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning.

Phosphorine is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent [C5H5BH]−.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Prismane or 'Ladenburg benzene' is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are arranged in the shape of a six-atom triangular prism—this compound is the parent and simplest member of the prismanes class of molecules. Albert Ladenburg proposed this structure for the compound now known as benzene. The compound was not synthesized until 1973.
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit and a chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of strained organic compounds.

In chemistry, pi stacking refers to the presumptive attractive, noncovalent pi interactions between the pi bonds of aromatic rings. However this is a misleading description of the phenomena since direct stacking of aromatic rings is electrostatically repulsive. What is more commonly observed is either a staggered stacking or pi-teeing interaction both of which are electrostatic attractive For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
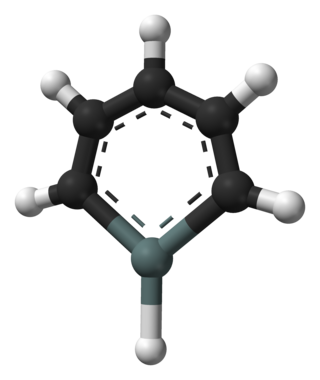
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolated.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.
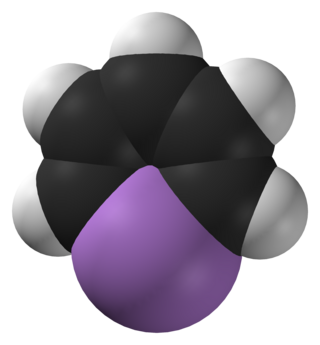
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).
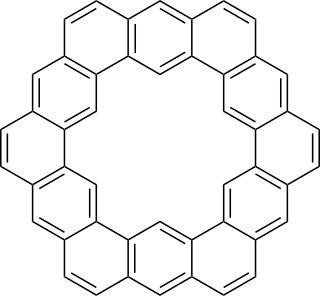
Kekulene is a polycyclic aromatic hydrocarbon which consists of 12 fused benzene rings arranged in a circle. It is therefore classified as a [12]-circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.
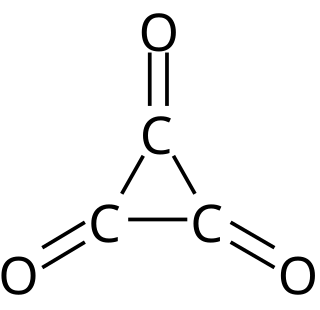
Cyclopropanetrione or trioxocyclopropane is a little-known oxide of carbon with formula C3O3. It consists of a ring of three carbon atoms each attached to an oxygen atom with a double bond. Alternately, it can be thought as a trimer of carbon monoxide. This compound is thermodynamically unstable and has not been produced in bulk. However, it has been detected using mass spectrometry.

Selenopyrylium is an aromatic heterocyclic compound consisting of a six-membered ring with five carbon atoms and a positively charged selenium atom.

Telluropyrylium is an aromatic heterocyclic compound consisting of a six member ring with five carbon atoms, and a positively charged tellurium atom. Derivatives of telluropyrylium are important in research of infrared dyes.

Stibinin, also known as stibabenzene, is an organic chemical compound. Stibinin has the chemical formula C5H5Sb. The molecule, stibinin, is a derivative of benzene, with one of the carbon atoms in the 6-membered ring replaced by an antimony (Sb) atom. Stibinin is a molecule that is considered to be an organoantimony compound due to it containing carbon, hydrogen, and antimony atoms.


















