
Thioredoxin reductases are the only known enzymes to reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond.

Glutathione S-transferases (GSTs), previously known as ligandins, comprise a family of eukaryotic and prokaryotic phase II metabolic isozymes best known for their ability to catalyze the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. The GST family consists of three superfamilies: the cytosolic, mitochondrial, and microsomal—also known as MAPEG—proteins. Members of the GST superfamily are extremely diverse in amino acid sequence, and a large fraction of the sequences deposited in public databases are of unknown function. The Enzyme Function Initiative (EFI) is using GSTs as a model superfamily to identify new GST functions.

Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.

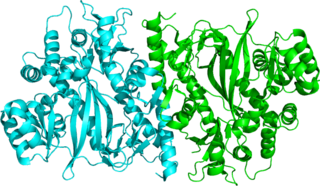
Glutathione reductase (GR) also known as glutathione-disulfide reductase (GSR) is an enzyme that in humans is encoded by the GSR gene. Glutathione reductase catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH), which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell. Glutathione reductase functions as dimeric disulfide oxidoreductase and utilizes an FAD prosthetic group and NADPH to reduce one molar equivalent of GSSG to two molar equivalents of GSH:
Glutathione synthetase (GSS) is the second enzyme in the glutathione (GSH) biosynthesis pathway. It catalyses the condensation of gamma-glutamylcysteine and glycine, to form glutathione. Glutathione synthetase is also a potent antioxidant. It is found in many species including bacteria, yeast, mammals, and plants.

Glutaredoxins are small redox enzymes of approximately one hundred amino-acid residues that use glutathione as a cofactor. In humans this oxidation repair enzyme is also known to participate in many cellular functions, including redox signaling and regulation of glucose metabolism. Glutaredoxins are oxidized by substrates, and reduced non-enzymatically by glutathione. In contrast to thioredoxins, which are reduced by thioredoxin reductase, no oxidoreductase exists that specifically reduces glutaredoxins. Instead, glutaredoxins are reduced by the oxidation of glutathione. Oxidized glutathione is then regenerated by glutathione reductase. Together these components compose the glutathione system.

In molecular biology, Adenylosuccinate synthase is an enzyme that plays an important role in purine biosynthesis, by catalysing the guanosine triphosphate (GTP)-dependent conversion of inosine monophosphate (IMP) and aspartic acid to guanosine diphosphate (GDP), phosphate and N(6)-(1,2-dicarboxyethyl)-AMP. Adenylosuccinate synthetase has been characterised from various sources ranging from Escherichia coli to vertebrate tissues. In vertebrates, two isozymes are present: one involved in purine biosynthesis and the other in the purine nucleotide cycle.
Arsenate reductase (glutaredoxin) (EC 1.20.4.1) is an enzyme that catalyzes the chemical reaction
Adenylyl-sulfate reductase (glutathione) is an enzyme that catalyzes the chemical reaction

In enzymology, an ATP phosphoribosyltransferase is an enzyme that catalyzes the chemical reaction

Glutathione S-transferase, C-terminal domain is a structural domain of glutathione S-transferase (GST).
In enzymology, a DNA beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction in which a beta-D-glucosyl residue is transferred from UDP-glucose to an hydroxymethylcytosine residue in DNA. It is analogous to the enzyme DNA alpha-glucosyltransferase.
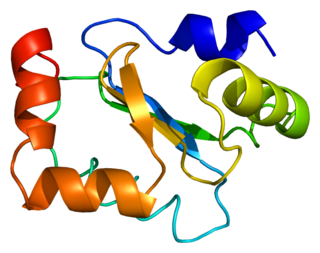
Glutaredoxin-1 is a protein that in humans is encoded by the GLRX gene.

Glutathione S-transferase Zeta 1 is an enzyme that in humans is encoded by the GSTZ1 gene on chromosome 14.
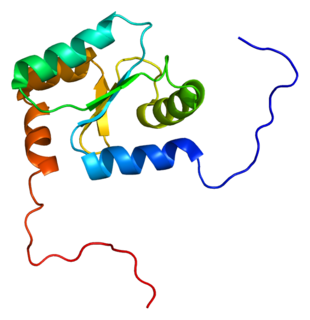
Glutaredoxin 2 (GLRX2) is an enzyme that in humans encoded by the GLRX2 gene. GLRX2, also known as GRX2, is a glutaredoxin family protein and a thiol-disulfide oxidoreductase that maintains cellular thiol homeostasis. This gene consists of four exons and three introns, spanned 10 kilobase pairs, and localized to chromosome 1q31.2–31.3.
Foyer-Halliwell-Asada pathway
Thioredoxins are small disulfide-containing redox proteins that have been found in all the kingdoms of living organisms. Thioredoxin serves as a general protein disulfide oxidoreductase. It interacts with a broad range of proteins by a redox mechanism based on reversible oxidation of 2 cysteine thiol groups to a disulfide, accompanied by the transfer of 2 electrons and 2 protons. The net result is the covalent interconversion of a disulfide and a dithiol.

In molecular biology, the ars operon is an operon found in several bacterial taxon. It is required for the detoxification of arsenate, arsenite, and antimonite. This system transports arsenite and antimonite out of the cell. The pump is composed of two polypeptides, the products of the arsA and arsB genes. This two-subunit enzyme produces resistance to arsenite and antimonite. Arsenate, however, must first be reduced to arsenite before it is extruded. A third gene, arsC, expands the substrate specificity to allow for arsenate pumping and resistance. ArsC is an approximately 150-residue arsenate reductase that uses reduced glutathione (GSH) to convert arsenate to arsenite with a redox active cysteine residue in the active site. ArsC forms an active quaternary complex with GSH, arsenate, and glutaredoxin 1 (Grx1). The three ligands must be present simultaneously for reduction to occur.
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.
The Monovalent Cation:Proton Antiporter-2 (CPA2) Family is a moderately large family of transporters belonging to the CPA superfamily. Members of the CPA2 family have been found in bacteria, archaea and eukaryotes. The proteins of the CPA2 family consist of between 333 and 900 amino acyl residues and exhibit 10-14 transmembrane α-helical spanners (TMSs).













