
Organ donation is the process when a person authorizes an organ of their own to be removed and transplanted to another person, legally, either by consent while the donor is alive, through a legal authorization for deceased donation made prior to death, or for deceased donations through the authorization by the legal next of kin.

William Harrison Frist is an American physician, businessman, conservationist and policymaker who served as a United States Senator from Tennessee from 1995 to 2007. A member of the Republican Party, he also served as Senate Majority Leader from 2003 to 2007. Born in Nashville, Tennessee, Frist studied government and health care policy at Princeton University and earned a Doctor of Medicine degree from Harvard Medical School. He trained as a cardiothoracic transplant surgeon at Massachusetts General Hospital and Stanford University School of Medicine, and later founded the Vanderbilt Transplant Center. In 1994, he defeated incumbent Democratic Senator Jim Sasser.
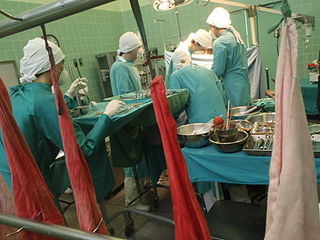
Organ transplantation is a medical procedure in which an organ is removed from one body and placed in the body of a recipient, to replace a damaged or missing organ. The donor and recipient may be at the same location, or organs may be transported from a donor site to another location. Organs and/or tissues that are transplanted within the same person's body are called autografts. Transplants that are recently performed between two subjects of the same species are called allografts. Allografts can either be from a living or cadaveric source.
The Uniform Anatomical Gift Act (UAGA), and its periodic revisions, is one of the Uniform Acts drafted by the National Conference of Commissioners on Uniform State Laws (NCCUSL), also known as the Uniform Law Commission (ULC), in the United States with the intention of harmonizing state laws between the states.

The 104th United States Congress was a meeting of the legislative branch of the United States federal government, composed of the United States Senate and the United States House of Representatives. It met in Washington, D.C. from January 3, 1995, to January 3, 1997, during the third and fourth years of Bill Clinton's presidency. Apportionment of seats in the House of Representatives was based on the 1990 United States census.

The Health Resources and Services Administration (HRSA) is an agency of the U.S. Department of Health and Human Services located in North Bethesda, Maryland. It is the primary federal agency for improving access to health care services for people who are uninsured, isolated or medically vulnerable.

Body donation, anatomical donation, or body bequest is the donation of a whole body after death for research and education. There is usually no cost to donate a body to science; donation programs will often provide a stipend and/or cover the cost of cremation or burial once a donated cadaver has served its purpose and is returned to the family for interment.

The United Network for Organ Sharing (UNOS) is a non-profit scientific and educational organization that administers the only Organ Procurement and Transplantation Network (OPTN) in the United States, established by the U.S. Congress in 1984 by Gene A. Pierce, founder of United Network for Organ Sharing. Located in Richmond, Virginia, the organization's headquarters are situated near the intersection of Interstate 95 and Interstate 64 in the Virginia BioTechnology Research Park.
Organ procurement is a surgical procedure that removes organs or tissues for reuse, typically for organ transplantation.

The National Organ Transplant Act (NOTA) of 1984 is an Act of the United States Congress that created the framework for the organ transplant system in the country. The act provided clarity on the property rights of human organs obtained from deceased individuals and established a public-private partnership known as Organ Procurement and Transplantation Network (OPTN). The OPTN was given the authority to oversee the national distribution of organs.
The Healthcare Systems Bureau is part of the Health Resources and Services Administration (HRSA), of the United States Department of Health and Human Services.
Elizabeth M. Duke was the administrator for the Health Resources and Services Administration (HRSA) from March 6, 2002 to February 28, 2009.
Many countries have laws, regulations, or recommendations that effectively prohibit donations of blood or tissue for organ and corneal transplants from men who have sex with men (MSM), a classification irrespective of their sexual activities with same-sex partners and of whether they identify themselves as bisexual or gay. Temporary restrictions are sometimes called "deferrals", since blood donors who are found ineligible may be found eligible at a later date. However, many deferrals are indefinite meaning that donations are not accepted at any point in the future, constituting a de facto ban. Even men who have monogamous relations with their same-sex partners are found ineligible.

The Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 is a law enacted by the 113th United States Congress. The Act amends the Public Health Service Act in order to extend, fund, and improve several programs designed to prepare the United States and health professionals in the event of a pandemic, epidemic, or biological, chemical, radiological, or nuclear accident or attack. The Act clarifies the authority of different American officials, makes it easier to temporarily reassign personnel to respond to emergency situations, and alters the process for testing and producing medical countermeasures. The Act is focused on improving preparedness for any public health emergency.

The Prematurity Research Expansion and Education for Mothers who deliver Infants Early Reauthorization Act or PREEMIE Reauthorization Act is a bill that reauthorizes research programs on preterm births that are run by the Centers for Disease Control and Prevention. It also authorizes grants and demonstration programs to be run by the Health Resources and Services Administration that will try to decrease preterm births. The bill passed the United States Senate during the 113th United States Congress.

The Child Care and Development Block Grant Act of 2013 is a bill that would reauthorize the Child Care and Development Block Grant Act of 1990 to provide block grants to the states to help low-income parents find child care for their children. In addition to reauthorizing the program, it also makes amendments to the law to try to improve it. Some of those improvements include required background checks on grant recipients and annual inspections.

Gregory Lyons Segal is an American entrepreneur.
Allegations of forced organ harvesting from Falun Gong practitioners and other political prisoners in China have raised concern within the international community. According to a report by former lawmaker David Kilgour, human rights lawyer David Matas and journalist Ethan Gutmann of the Victims of Communism Memorial Foundation, political prisoners, mainly Falun Gong practitioners, are being executed "on demand" in order to provide organs for transplant to recipients. Reports have said that organ harvesting has been used to advance the Chinese Communist Party's persecution of Falun Gong and because of the financial incentives available to the institutions and individuals involved in the trade. A report by The Washington Post has disputed some of the allegations, saying that China does not import sufficient quantities of immunosuppressant drugs, used by transplant recipients, to carry out such quantities of organ harvesting. However, a group of experts stated that the Post's article made an “elementary statistical error” and omitted unofficial pharmacy data in Chinese hospitals.

Dorry L. Segev is the Marjory K. and Thomas Pozefsky Professor of Surgery at Johns Hopkins School of Medicine, professor of epidemiology at Johns Hopkins Bloomberg School of Public Health, and associate vice chair of the Department of Surgery at Johns Hopkins Hospital. He has made significant contributions to the field of transplantation, including developing a mathematical model to facilitate a nationwide kidney paired donation program, both in the US and Canada. He is also known for his role in getting the HIV Organ Policy Equity Act signed into law.

Jenna Arnold is an American activist, entrepreneur and author of Raising Our Hands (2020). She is known as the co-founder of ORGANIZE, for her work at the United Nations and MTV, and was a National Organizer for the 2017 Women's March on Washington. Oprah has called Arnold one of the "100 Awakened Leaders who are using their voice and talent to elevate humanity". She is a frequent contributor on the subjects of American identity, politics and foreign policy on FOX, CNN, and MSNBC.











