
The beta sheet, (β-sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease.
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common three-dimensional structure that appears in a variety of different, evolutionarily unrelated molecules. A structural motif does not have to be associated with a sequence motif; it can be represented by different and completely unrelated sequences in different proteins or RNA.

In molecular biology, pertactin (PRN) is a highly immunogenic virulence factor of Bordetella pertussis, the bacterium that causes pertussis. Specifically, it is an outer membrane protein that promotes adhesion to tracheal epithelial cells. PRN is purified from Bordetella pertussis and is used for the vaccine production as one of the important components of acellular pertussis vaccine.

Bordetella is a genus of small, Gram-negative, coccobacilli bacteria of the phylum Pseudomonadota. Bordetella species, with the exception of B. petrii, are obligate aerobes, as well as highly fastidious, or difficult to culture. All species can infect humans. The first three species to be described ; are sometimes referred to as the 'classical species'. Two of these are also motile.
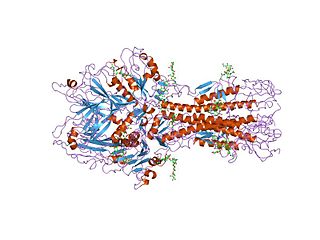
Hemagglutinin esterase (HEs) is a glycoprotein that certain enveloped viruses possess and use as an invading mechanism. HEs helps in the attachment and destruction of certain sialic acid receptors that are found on the host cell surface. Viruses that possess HEs include influenza C virus, toroviruses, and coronaviruses of the subgenus Embecovirus. HEs is a dimer transmembrane protein consisting of two monomers, each monomer is made of three domains. The three domains are: membrane fusion, esterase, and receptor binding domains.
Adhesins are cell-surface components or appendages of bacteria that facilitate adhesion or adherence to other cells or to surfaces, usually in the host they are infecting or living in. Adhesins are a type of virulence factor.
A DNA-binding domain (DBD) is an independently folded protein domain that contains at least one structural motif that recognizes double- or single-stranded DNA. A DBD can recognize a specific DNA sequence or have a general affinity to DNA. Some DNA-binding domains may also include nucleic acids in their folded structure.

Bordetella pertussis is a Gram-negative, aerobic, pathogenic, encapsulated coccobacillus of the genus Bordetella, and the causative agent of pertussis or whooping cough. Like B. bronchiseptica, B. pertussis is motile and expresses a flagellum-like structure. Its virulence factors include pertussis toxin, adenylate cyclase toxin, filamentous hæmagglutinin, pertactin, fimbria, and tracheal cytotoxin.

The TIM barrel, also known as an alpha/beta barrel, is a conserved protein fold consisting of eight alpha helices (α-helices) and eight parallel beta strands (β-strands) that alternate along the peptide backbone. The structure is named after triose-phosphate isomerase, a conserved metabolic enzyme. TIM barrels are ubiquitous, with approximately 10% of all enzymes adopting this fold. Further, five of seven enzyme commission (EC) enzyme classes include TIM barrel proteins. The TIM barrel fold is evolutionarily ancient, with many of its members possessing little similarity today, instead falling within the twilight zone of sequence similarity.
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Bordetella bronchiseptica and Bordetella parapertussis, also able to cause pertussis-like symptoms, also produce adenylate cyclase toxin. It is a toxin secreted by the bacteria to influence the host immune system.

A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand-turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.
The Kelch motif is a region of protein sequence found widely in proteins from bacteria and eukaryotes. This sequence motif is composed of about 50 amino acid residues which form a structure of a four stranded beta-sheet "blade". This sequence motif is found in between five and eight tandem copies per protein which fold together to form a larger circular solenoid structure called a beta-propeller domain.
Adenylate cyclase toxin (CyaA) is released from bacterium Bordetella pertussis by the T1SS and released in the host’s respiratory tract in order to suppress its early innate and subsequent adaptive immune defense.
The filamentous haemagglutinin adhesin (FHA) is a large, filamentous protein that serves as a dominant attachment factor for adherence to host ciliated epithelial cells of the respiratory tract, called respiratory epithelium. It is associated with biofilm formation and possesses at least four binding domains which can bind to different cell receptors on the epithelial cell surface. One notable bacterium that produces filamentous haemagglutinin adhesin is Bordetella pertussis, which uses this protein as a virulence factor.

In molecular biology, the protein domain SdrG C terminal refers to the C terminus domain of an adhesin found only on the cell walls of bacteria. More specifically, SdrG is only found in gram-positive bacteria. This particular domain binds to a glycoprotein named fibrinogen. SdrG stands for serine-aspartate dipeptide repeats, which as its name suggests, contains repeats of two amino acids, serine and aspartate.

In molecular biology the B-box-type zinc finger domain is a short protein domain of around 40 amino acid residues in length. B-box zinc fingers can be divided into two groups, where types 1 and 2 B-box domains differ in their consensus sequence and in the spacing of the 7-8 zinc-binding residues. Several proteins contain both types 1 and 2 B-boxes, suggesting some level of cooperativity between these two domains.
In molecular biology, the CVNH domain is a conserved protein domain. It is found in the sugar-binding antiviral protein cyanovirin-N (CVN) as well as proteins from filamentous ascomycetes and in the fern Ceratopteris richardii.

In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria. Bacteria use TAAs in order to infect their host cells via a process called cell adhesion. TAAs also go by another name, oligomeric coiled-coil adhesins, which is shortened to OCAs. In essence, they are virulence factors, factors that make the bacteria harmful and infective to the host organism.

In molecular biology, YadA is a protein domain which is short for Yersinia adhesin A. These proteins have strong sequence and structural homology, particularly at their C-terminal end. The function is to promote their pathogenicity and virulence in host cells, though cell adhesion. YadA is found in three pathogenic species of Yersinia, Y. pestis,Y. pseudotuberculosis, and Y. enterocolitica. The YadA domain is encoded for by a virulence plasmid in Yersinia, which encodes a type-III secretion (T3S) system consisting of the Ysc injectisome and the Yop effectors.













