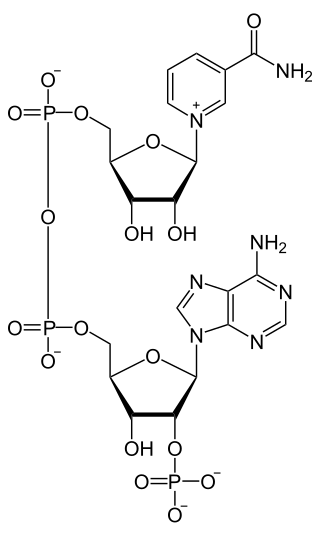
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.
Antimycins are produced as secondary metabolites by Streptomyces bacteria, a soil bacteria. These specialized metabolites likely function to kill neighboring organisms in order to provide the streptomyces bacteria with a competitive edge.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.
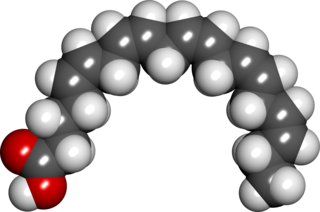
Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5(n-3). It also has the trivial name timnodonic acid. In chemical structure, EPA is a carboxylic acid with a 20-carbon chain and five cis double bonds; the first double bond is located at the third carbon from the omega end.
The Carroll rearrangement is a rearrangement reaction in organic chemistry and involves the transformation of a β-keto allyl ester into a α-allyl-β-ketocarboxylic acid. This organic reaction is accompanied by decarboxylation and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement and effectively a decarboxylative allylation.

Platensimycin, a metabolite of Streptomyces platensis, is an antibiotic, which act by blocking enzymes.

Prodigiosin is the red dyestuff produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginines family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells.

Lipstatin is a potent, irreversible inhibitor of pancreatic lipase. It is a natural product that was first isolated from the actinobacterium Streptomyces toxytricini.

In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis. It typically uses malonyl-CoA as a carbon source to elongate ACP-bound acyl species, resulting in the formation of ACP-bound β-ketoacyl species such as acetoacetyl-ACP.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
In enzymology, a long-chain-alcohol O-fatty-acyltransferase is an enzyme that catalyzes the chemical reaction

In organic chemistry, keto acids or ketoacids are organic compounds that contain a carboxylic acid group and a ketone group. In several cases, the keto group is hydrated. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis.
Curcumin synthase categorizes three enzyme isoforms, type III polyketide synthases (PKSs) present in the leaves and rhizome of the turmeric plant that synthesize curcumin. CURS1-3 are responsible for the hydrolysis of feruloyldiketide-CoA, previously produced in the curcuminoid pathway, and a decarboxylative condensation reaction that together comprise one of the final steps in the synthesis pathway for curcumin, demethoxycurcumin, and bisdemethoxycurcumin, the compounds that give turmeric both its distinctive yellow color, and traditional medical benefits. CURS should not be confused with Curcuminoid Synthase (CUS), which catalyzes the one-pot synthesis of bisdemethoxycurcumin in Oryza sativa.

Modified aldol tandem reaction is a sequential chemical transformation that combines aldol reaction with other chemical reactions that generate enolates. Enolates are a common building block in chemical syntheses and are typically formed by the addition of base to a ketone or aldehyde. Modified Aldol tandem reactions allow similar reactivity to be produced without the need for a base which may have adverse effects in a given chemical synthesis. A representative example is the decarboxylative aldol reaction, where the enolate is generated via decarboxylation reaction mediated by either transition metals or organocatalysts. Key advantage of this reaction over other types of aldol reaction is the selective generation of an enolate in the presence of aldehydes. This allows for the directed aldol reaction to produce a desired cross aldol.

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.
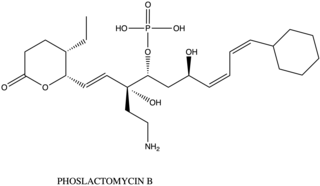
Phoslactomycin (PLM) is a natural product from the isolation of Streptomyces species. This is an inhibitor of the protein serine/threonine phosphatase which is the protein phosphate 2A (PP2A). The PP2A involves the growth factor of the cell such as to induce the formation of mitogen-activated protein interaction and playing a role in cell division and signal transduction. Therefore, PLM is used for the drug that prevents the tumor, cancer, or bacteria. There are nowsaday has 7 kinds of different PLM from PLM A to PLM G which differ the post-synthesis from the biosynthesis of PLM.

Pyonitrins are a family of highly hydrogen-deficient alkaloids discovered from an insect-associated Pseudomonas protegens strain. In vivo, pyonitrins A-D show activity against pathogen Candida albicans, which commonly cause bloodstream infections.
Hydrocarbonoclastic bacteria are a heterogeneous group of prokaryotes which can degrade and utilize hydrocarbon compounds as source of carbon and energy. Despite being present in most of environments around the world, several of these specialized bacteria live in the sea and have been isolated from polluted seawater.












