
Cinnamaldehyde is an organic compound with the formula C6H5CH=CHCHO. Occurring naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde decomposes to styrene because of oxidation as a result of bad storage or transport conditions. Styrene especially forms in high humidity and high temperatures. This is the reason why cinnamon contains small amounts of styrene.

Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes are not contagious or life-threatening, but can be very uncomfortable.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

Nummular dermatitis is one of the many forms of dermatitis. it is characterized by round or oval-shaped itchy lesions. The name comes from the Latin word "nummus," which means "coin."
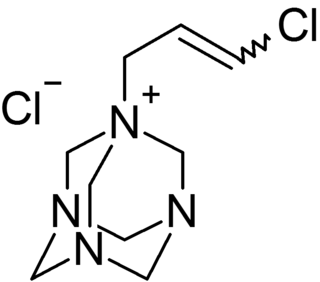
Quaternium-15 is a quaternary ammonium salt used as a surfactant and preservative in many cosmetics and industrial substances. It acts as an antimicrobial agent because it acts as a formaldehyde releaser, though doing so can also cause contact dermatitis, a symptom of an allergic reaction, especially in those with sensitive skin.

Allergic contact dermatitis (ACD) is a form of contact dermatitis that is the manifestation of an allergic response caused by contact with a substance; the other type being irritant contact dermatitis (ICD).

Balsam of Peru or Peru balsam, also known and marketed by many other names, is a balsam derived from a tree known as Myroxylon balsamum var. pereirae; it is found in El Salvador, where it is an endemic species.
Hydroxymethylpentylcyclohexenecarboxaldehyde is a synthetic fragrance known by the trade names Lyral, Kovanol, Mugonal, Landolal. It is found in some soaps, eau de toilettes, aftershaves and deodorants.

Tetrazepam is a benzodiazepine derivative with anticonvulsant, anxiolytic, muscle relaxant and slightly hypnotic properties. It was formerly used mainly in Austria, France, Belgium, Germany and Spain to treat muscle spasm, anxiety disorders such as panic attacks, or more rarely to treat depression, premenstrual syndrome or agoraphobia. Tetrazepam has relatively little sedative effect at low doses while still producing useful muscle relaxation and anxiety relief. The Co-ordination Group for Mutual Recognition and Decentralised Procedures-Human endorsed the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation to suspend the marketing authorisations of tetrazepam-containing medicines across the European Union (EU) in April 2013. The European Commission has confirmed the suspension of the marketing authorisations for Tetrazepam in Europe because of cutaneous toxicity, effective from the 1 August 2013.

Diazolidinyl urea is an antimicrobial preservative used in cosmetics. It is chemically related to imidazolidinyl urea which is used in the same way. Diazolidinyl urea acts as a formaldehyde releaser.

Phenylmercuric acetate is an organomercury compound. This compound was formerly used as a preservative in paints, and as a disinfectant. When applied to the leaves of plants, it is an antitranspirant.

DMDM hydantoin is an antimicrobial formaldehyde releaser preservative with the trade name Glydant. DMDM hydantoin is an organic compound belonging to a class of compounds known as hydantoins. It is used in the cosmetics industry and found in products like shampoos, hair conditioners, hair gels, and skin care products.
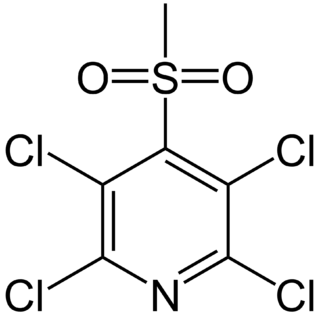
Davicil is a chlorinated pyridine derivative with antimicrobial properties, which is used as a fungicide. It can be allergenic in humans and produce contact dermatitis.
Perfume intolerance or perfume allergy is a condition wherein people exhibit sensitivity or allergic reactions to ingredients in some perfumes and some other fragrances. It is a form of multiple chemical sensitivity, a more general phenomenon for this diagnosis.
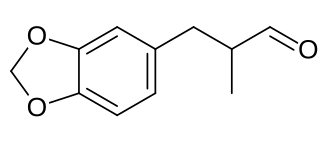
Helional is a chemical compound used as a perfume in soap and laundry detergent. Chemically it is an aldehyde with a hydrocinnamaldehyde motif; a structural element which is present in a number of other important commercial fragrances and odorants.

Iodopropynyl Butyl Carbamate (IPBC) is a water-soluble preservative used globally in the paints & coatings, wood preservatives, personal care, and cosmetics industries. IPBC is a member of the carbamate family of biocides. IPBC was invented in the 1970s and has a long history of effective use as an antifungal technology.
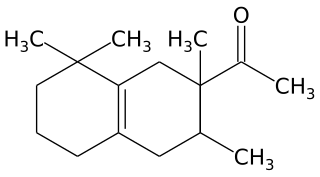
Tetramethyl acetyloctahydronaphthalenes is a synthetic ketone fragrance also known as OTNE and by other commercial trade names such as: Iso E Super, Iso Gamma Super, Anthamber, Amber Fleur, Boisvelone, Iso Ambois, Amberlan, Iso Velvetone, Orbitone, Amberonne. It is a synthetic woody odorant and is used as a fragrance ingredient in perfumes, laundry products and cosmetics.

Nickel allergy or nickel allergic contact dermatitis (Ni-ACD) is a form of allergic contact dermatitis (ACD) caused by exposure to the chemical element nickel. It typically causes a rash that is red and itchy and that may be bumpy or scaly. The main treatment is avoiding contact with nickel-releasing metals, such as wearing inexpensive jewelry.
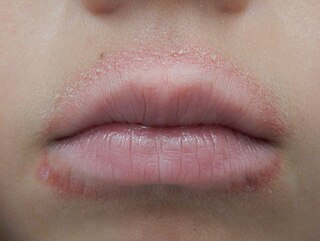
Lip licker's dermatitis is a type of skin inflammation around the lips due to damage by saliva from repetitive lip licking and is classified as a subtype of irritant contact cheilitis. The resulting scaling, redness, chapping, and crusting makes a well-defined ring around the lips. The rash may extend as far as the tongue can reach and usually does not occur at the corners of the mouth. It commonly occurs during winter months but some people can have it year-round if lip licking is a chronic habit.
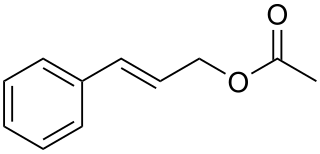
Cinnamyl acetate is a chemical compound of the cinnamyl ester family, in which the variable R group is substituted by a methyl group. As a result of the non-aromatic carbon-carbon double bond, cinnamyl acetate can exist in a Z and an E configuration:

















