
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

Sulfuric acid or sulphuric acid, known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water.
An electrolyte is a medium containing ions that are electrically conductive through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood.

Weathering is the deterioration of rocks, soils and minerals through contact with water, atmospheric gases, sunlight, and biological organisms. Weathering occurs in situ, and so is distinct from erosion, which involves the transport of rocks and minerals by agents such as water, ice, snow, wind, waves and gravity.

In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mild dehydration can also be caused by immersion diuresis, which may increase risk of decompression sickness in divers.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate Na
2SiO
3, sodium orthosilicate Na
4SiO
4, and sodium pyrosilicate Na
6Si
2O
7. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.

Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, and Roman vitriol. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Metasomatism is the chemical alteration of a rock by hydrothermal and other fluids. It is traditionally defined as metamorphism which involves a change in the chemical composition, excluding volatile components. It is the replacement of one rock by another of different mineralogical and chemical composition. The minerals which compose the rocks are dissolved and new mineral formations are deposited in their place. Dissolution and deposition occur simultaneously and the rock remains solid.
A heating pad is a pad used for warming of parts of the body in order to manage pain. Localized application of heat causes the blood vessels in that area to dilate, enhancing perfusion to the targeted tissue. Types of heating pads include electrical, chemical and hot water bottles.

A hydration pack or drink bag is a type of hydration system built as a backpack or waistpack containing a reservoir or "bladder" commonly made of rubber or flexible plastic. The reservoir contains a capped mouth for filling with liquid and a hose that allows the wearer to drink hands-free. Most hoses end with a "bite valve" that opens when the user bites down on it; the valve may be protected by a dust cover. Some hydration packs are insulated to keep water from freezing or becoming warm.

Embalming chemicals are a variety of preservatives, sanitising and disinfectant agents, and additives used in modern embalming to temporarily prevent decomposition and restore a natural appearance for viewing a body after death. A mixture of these chemicals is known as embalming fluid and is used to preserve bodies of deceased persons for both funeral purposes and in medical research in anatomical laboratories. The period for which a body is embalmed is dependent on time, expertise of the embalmer and factors regarding duration of stay and purpose.
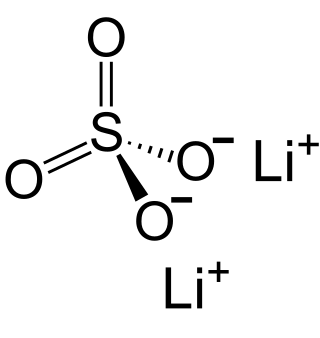
Lithium sulfate is a white inorganic salt with the formula Li2SO4. It is the lithium salt of sulfuric acid.

Sports nutrition is the study and practice of nutrition and diet with regards to improving anyone's athletic performance. Nutrition is an important part of many sports training regimens, being popular in strength sports and endurance sports. Sports nutrition focuses its studies on the type, as well as the quantity of fluids and food taken by an athlete. In addition, it deals with the consumption of nutrients such as vitamins, minerals, supplements and organic substances that include carbohydrates, proteins and fats.
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor.

The following outline is provided as an overview of and topical guide to water:
Tissue hydration is the process of absorbing and retaining water in biological tissues.

Leonite is a hydrated double sulfate of magnesium and potassium. It has the formula K2SO4·MgSO4·4H2O. The mineral was named after Leo Strippelmann, who was director of the salt works at Westeregeln in Germany. The mineral is part of the blodite group of hydrated double sulfate minerals.

Neodymium(III) acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.













