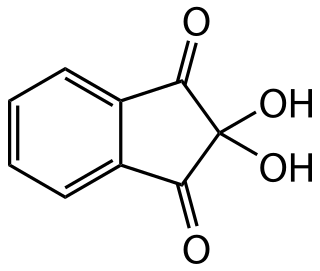
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is an organic compound with the formula C6H4(CO)2C(OH)2. It is used to detect ammonia and amines. Upon reaction with these amines, ninhydrin gets converted into deep blue or purple derivatives, which are called Ruhemann's purple. Ninhydrin is most commonly used to detect fingerprints in forensic cases, as the terminal amines of lysine residues in peptides and proteins sloughed off in fingerprints react with ninhydrin.
Inborn errors of metabolism form a large class of genetic diseases involving congenital disorders of enzyme activities. The majority are due to defects of single genes that code for enzymes that facilitate conversion of various substances (substrates) into others (products). In most of the disorders, problems arise due to accumulation of substances which are toxic or interfere with normal function, or due to the effects of reduced ability to synthesize essential compounds. Inborn errors of metabolism are often referred to as congenital metabolic diseases or inherited metabolic disorders. Another term used to describe these disorders is "enzymopathies". This term was created following the study of biodynamic enzymology, a science based on the study of the enzymes and their products. Finally, inborn errors of metabolism were studied for the first time by British physician Archibald Garrod (1857–1936), in 1908. He is known for work that prefigured the "one gene–one enzyme" hypothesis, based on his studies on the nature and inheritance of alkaptonuria. His seminal text, Inborn Errors of Metabolism, was published in 1923.
Kaiser is the German word for "emperor", usually reserved for the emperors of the German and Austrian Empires.

In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
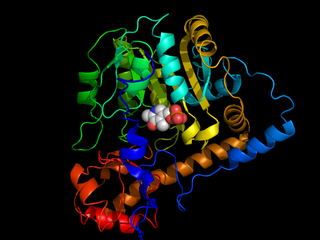
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
The D-500 Amino Acid Analyzer was designed and built by Durrum Instruments in the late 1960s. It was used by many prestigious universities and research facilities to test for presence and quantity of amino acids in biological samples. At that time, Durrum was located on Fabian Way, Palo Alto, California, which was the birthplace of many high-tech companies. The D-500 operates using ion exchange chromatography.

Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant, for which it is named, as well as in the common walnut and water hyacinth. Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5,000 years. Lawsone reacts chemically with the protein keratin in skin and hair via a Michael addition reaction, resulting in a strong permanent stain that lasts until the skin or hair is shed. Darker colored staining is due to more lawsone–keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning agents and sunscreens. Lawsone is a 1,4-naphthoquinone derivative, an analog of hydroxyquinone containing one additional ring.

Hawkinsin (also known as 2-cystenyl-1,4-dihydroxycyclohexenylacetate) is an amino acid, which is formed after detoxification of a reactive tyrosine metabolite (quinol acetate) by glutathione. Hawkinsin is ninhydrin positive (a common test to detect amino acids and proteins with a free -NH2 group).

Millon's reagent is an analytical reagent used to detect the presence of soluble proteins. A few drops of the reagent are added to the test solution, which is then heated gently. A reddish-brown coloration or precipitate indicates the presence of tyrosine residue which occur in nearly all proteins. The test was developed by the French chemist Auguste Nicolas Eugene Millon.
Phosphoamino acid analysis, or PAA, is an experimental technique used in molecular biology to determine which amino acid or acids are phosphorylated in a protein.
The molecular formula C9H6O4 (molar mass: 178.14 g/mol, exact mass 178.026609 u) may refer to:

Glove prints, also sometimes described as gloveprints or glove marks, are latent, fingerprint-like impressions that are transferred to a surface or object by an individual who is wearing gloves.

Madeleine M. Joullié is an American-Brazilian organic chemist. She was the first woman to join the University of Pennsylvania chemistry faculty as well as the first female organic chemist to be appointed to a tenure track position in a major American university. She was one of the first affirmative action officers at the University of Pennsylvania. She has a distinguished record as a teacher of both undergraduate and graduate-level organic chemistry, and as a mentor of students.
In organic chemistry, secondary amino acids are amino acids which do not contain the amino group −NH2 but is rather a secondary amine. Secondary amino acids can be classified to cyclic acids, such as proline, and acyclic N-substituted amino acids.
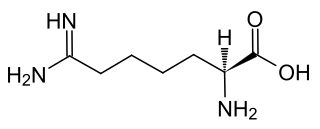
Indospicine is an amino acid not found in proteins, which occurs in Indigofera species. The chemical resembles arginine.

Indane-1,2,3-trione is the organic compound with the formula C6H4(CO)3. The compound is the dehydrated derivative of C6H4(CO)2C(OH)2, known as ninhydrin, which is used to reveal fingerprints.

Pauline Merritt Hald was an American clinical chemist and medical researcher, based in New Haven, Connecticut. She worked in the laboratory of chemist John P. Peters for many years, and published the first description of his flame photometry technique for measuring serum sodium and potassium levels.
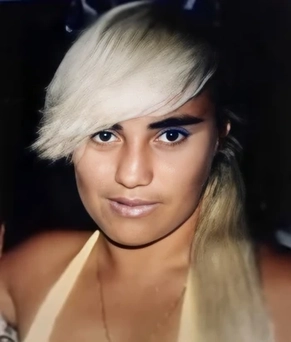
The murder of Vivianne Ruiz occurred in Sydney, Australia. Her body was discovered in garbage bags on 28 December 1991 without any identification. A postmortem found strangulation to be the likely cause of death. Animal hairs were found on the body, and newspaper was stuffed inside her mouth.
Colorogenic or colourogenic describes a property of chemical compounds which are initially not colored, but become colored through a chemical reaction, often through an intermolecular covalent reaction that either covalently binds the now colored compound to a target molecule, or through a reaction which leads to a colored non-covalently bound product. Colorogenic reagents are often used for qualitative testing for the presence of chemical functional groups. Colorogenic labeling reagents are sometimes used in analytical chemistry procedures, for example in HPLC or CE to derivative target compounds, however the enhanced sensitivity from the analogous fluorogenic reagents, leads colorogenic reagents to be less frequently used in quantitative applications.