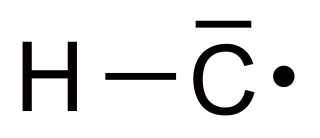Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science.

Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction made in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.

The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and stress corrosion cracking in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as synproportionation.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
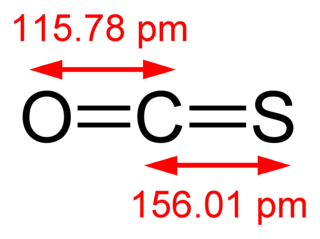
Carbonyl sulfide is the chemical compound with the linear formula O=C=S. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl double bonded to a sulfur atom. Carbonyl sulfide can be considered to be intermediate between carbon dioxide and carbon disulfide, both of which are valence isoelectronic with it.

The hydroperoxyl radical, also known as the hydrogen superoxide, is the protonated form of superoxide with the chemical formula HO2, also written HOO•. This species plays an important role in the atmosphere and as a reactive oxygen species in cell biology.
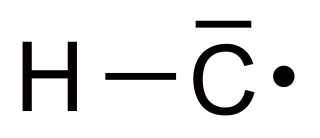
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen.
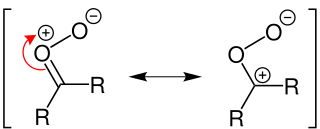
A Criegee intermediate is a carbonyl oxide with two charge centers. These chemicals may react with sulfur dioxide and nitrogen oxides in the Earth's atmosphere, and are implicated in the formation of aerosols, which are an important factor in controlling global climate. Criegee intermediates are also an important source of OH. OH radicals are the most important oxidant in the troposphere, and are important in controlling air quality and pollution.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.
Paul O. Wennberg is the R. Stanton Avery Professor of Atmospheric Chemistry and Environmental Science and Engineering at the California Institute of Technology (Caltech). He is the director of the Ronald and Maxine Linde Center for Global Environmental Science. He is chair of the Total Carbon Column Observing Network and a founding member of the Orbiting Carbon Observatory project, which created NASA's first spacecraft for analysis of carbon dioxide in the atmosphere. He is also the principal investigator for the Mars Atmospheric Trace Molecule Occultation Spectrometer (MATMOS) to investigate trace gases in Mars's atmosphere.
Sulfur mononitride is an inorganic compound with the molecular formula SN. It is the sulfur analogue of and isoelectronic to the radical nitric oxide, NO. It was initially detected in 1975, in outer space in giant molecular clouds and later the coma of comets. This spurred further laboratory studies of the compound. Synthetically, it is produced by electric discharge in mixtures of nitrogen and sulfur compounds, or combustion in the gas phase and by photolysis in solution.
Sulfanyl (HS•), also known as the mercapto radical, hydrosulfide radical, or hydridosulfur, is a simple radical molecule consisting of one hydrogen and one sulfur atom. The radical appears in metabolism in organisms as H2S is detoxified. Sulfanyl is one of the top three sulfur-containing gasses in gas giants such as Jupiter and is very likely to be found in brown dwarfs and cool stars. It was originally discovered by Margaret N. Lewis and John U. White at the University of California in 1939. They observed molecular absorption bands around 325 nm belonging to the system designated by 2Σ+ ← 2Πi. They generated the radical by means of a radio frequency discharge in hydrogen sulfide. HS• is formed during the degradation of hydrogen sulfide in the atmosphere of the Earth. This may be a deliberate action to destroy odours or a natural phenomenon.

Carbon pentaoxide, carbon pentoxide or tetraoxolan-5-one is an unstable molecular oxide of carbon. The molecule has been produced and studied at cryogenic temperatures. The molecule is important in atmospheric chemistry and in the study of cold ices in the outer solar system and interstellar space. The substance could form and be present on Ganymede or Triton, moons in the outer solar system.
A post column oxidation-reduction reactor is a chemical reactor that performs derivatization to improve the measurement of organic molecules. It is used in gas chromatography (GC), after the column, and before a flame ionization detector (FID), to make the detector response uniform for all organic molecules.

Tricarbon monoxide C3O is a reactive radical oxocarbon molecule found in space, and which can be made as a transient substance in the laboratory. It can be trapped in an inert gas matrix or made as a short lived gas. C3O can be classified as a ketene or an oxocumulene a kind of heterocumulene.