Acidosis is a biological process producing hydrogen ions and increasing their concentration in blood or body fluids. pH is the negative log of hydrogen ion concentration and so it is decreased by a process of acidosis.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

Hypovolemic shock is a form of shock caused by severe hypovolemia. It can be caused by severe dehydration or blood loss. Hypovolemic shock is a medical emergency; if left untreated, the insufficient blood flow can cause damage to organs, leading to multiple organ failure.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.

Ringer's lactate solution (RL), also known as sodium lactate solution,Lactated Ringer's (LR), and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area.
The anion gap is a value calculated from the results of multiple individual medical lab tests. It may be reported with the results of an electrolyte panel, which is often performed as part of a comprehensive metabolic panel.
Hypoaldosteronism is an endocrinological disorder characterized by decreased levels of the hormone aldosterone. Similarly, isolated hypoaldosteronism is the condition of having lowered aldosterone without corresponding changes in cortisol.

Metabolic alkalosis is an acid-base disorder in which the pH of tissue is elevated beyond the normal range (7.35–7.45). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. The condition typically cannot last long if the kidneys are functioning properly.
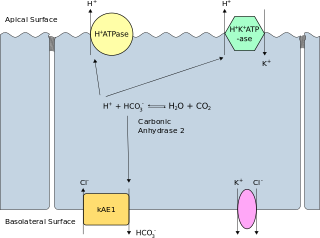
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
In physiology, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base present in the blood. The value is usually reported as a concentration in units of mEq/L (mmol/L), with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2 mEq/L.

Renal tubular acidosis (RTA) is a medical condition that involves an accumulation of acid in the body due to a failure of the kidneys to appropriately acidify the urine. In renal physiology, when blood is filtered by the kidney, the filtrate passes through the tubules of the nephron, allowing for exchange of salts, acid equivalents, and other solutes before it drains into the bladder as urine. The metabolic acidosis that results from RTA may be caused either by insufficient secretion of hydrogen ions into the latter portions of the nephron or by failure to reabsorb sufficient bicarbonate ions from the filtrate in the early portion of the nephron. Although a metabolic acidosis also occurs in those with chronic kidney disease, the term RTA is reserved for individuals with poor urinary acidification in otherwise well-functioning kidneys. Several different types of RTA exist, which all have different syndromes and different causes. RTA is usually an incidental finding based on routine blood draws that show abnormal results. Clinically, patients may present with vague symptoms such as dehydration, mental status changes, or delayed growth in adolescents.
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases in the ECF is crucial for the normal physiology of the body—and for cellular metabolism. The pH of the intracellular fluid and the extracellular fluid need to be maintained at a constant level.
Contraction alkalosis refers to the increase in blood pH that occurs as a result of fluid losses. The change in pH is especially pronounced with acidic fluid losses caused by problems like vomiting.

Renal compensation is a mechanism by which the kidneys can regulate the plasma pH. It is slower than respiratory compensation, but has a greater ability to restore normal values. Kidneys maintain the acid-base balance through two mechanisms: (1) the secretion of H+ ions into the urine (from the blood) and (2) the reabsorption of bicarbonate HCO−
3 (i.e., bicarbonate moves from urine back into the blood). The regulation of H+ ions and bicarbonate HCO−
3 is determined by the concentration of the two released within the urine. These mechanisms of secretion and reabsorption balance the pH of the bloodstream. A restored acid-base balanced bloodstream thus leads to a restored acid-base balance throughout the entire body.
Normal anion gap acidosis is an acidosis that is not accompanied by an abnormally increased anion gap.
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range. In the fetus, the normal range differs based on which umbilical vessel is sampled. It can exist in varying levels of severity, some life-threatening.
In clinical chemistry, the urine anion gap is calculated using measured ions found in the urine. It is used to aid in the differential diagnosis of metabolic acidosis.

Distal renal tubular acidosis (dRTA) is the classical form of RTA, being the first described. Distal RTA is characterized by a failure of acid secretion by the alpha intercalated cells of the distal tubule and cortical collecting duct of the distal nephron. This failure of acid secretion may be due to a number of causes. It leads to relatively alkaline urine, due to the kidney's inability to acidify the urine to a pH of less than 5.3.
In nephrology, the delta ratio, or "delta-delta", is a formula that can be used to evaluate whether a mixed acid–base disorder is present, and if so, assess its severity. The anion gap (AG) without potassium is calculated first and if a metabolic acidosis is present, results in either a high anion gap metabolic acidosis (HAGMA) or a normal anion gap acidosis (NAGMA). A low anion gap is usually an oddity of measurement, rather than a clinical concern.










