
Radiation therapy or radiotherapy is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist.

External beam radiation therapy (EBRT) is a form of radiotherapy that utilizes a high-energy collimated beam of ionizing radiation, from a source outside the body, to target and kill cancer cells. A radiotherapy beam is composed of particles which travel in a consistent direction; each radiotherapy beam consists of one type of particle intended for use in treatment, though most beams contain some contamination by other particle types.
A radiation therapist, therapeutic radiographer or radiotherapist is an allied health professional who works in the field of radiation oncology. Radiation therapists plan and administer radiation treatments to cancer patients in most Western countries including the United Kingdom, Australia, most European countries, and Canada, where the minimum education requirement is often a baccalaureate degree or postgraduate degrees in radiation therapy. Radiation therapists can also prescribe medications and radiation, interpret tests results, perform follow ups, reviews, and provide consultations to cancer patients in the United Kingdom and Ontario, Canada . In the United States, radiation therapists have a lower educational requirement and often require postgraduate education and certification in order to plan treatments.

The Paul Scherrer Institute (PSI) is a multi-disciplinary research institute for natural and engineering sciences in Switzerland. It is located in the Canton of Aargau in the municipalities Villigen and Würenlingen on either side of the River Aare, and covers an area over 35 hectares in size. Like ETH Zurich and EPFL, PSI belongs to the Swiss Federal Institutes of Technology Domain of the Swiss Confederation. The PSI employs around 2,100 people. It conducts basic and applied research in the fields of matter and materials, human health, and energy and the environment. About 37% of PSI's research activities focus on material sciences, 24% on life sciences, 19% on general energy, 11% on nuclear energy and safety, and 9% on particle physics.

In medicine, proton therapy, or proton radiotherapy, is a type of particle therapy that uses a beam of protons to irradiate diseased tissue, most often to treat cancer. The chief advantage of proton therapy over other types of external beam radiotherapy is that the dose of protons is deposited over a narrow range of depth; hence in minimal entry, exit, or scattered radiation dose to healthy nearby tissues.

Radiosurgery is surgery using radiation, that is, the destruction of precisely selected areas of tissue using ionizing radiation rather than excision with a blade. Like other forms of radiation therapy, it is usually used to treat cancer. Radiosurgery was originally defined by the Swedish neurosurgeon Lars Leksell as "a single high dose fraction of radiation, stereotactically directed to an intracranial region of interest".

The Bragg peak is a pronounced peak on the Bragg curve which plots the energy loss of ionizing radiation during its travel through matter. For protons, α-rays, and other ion rays, the peak occurs immediately before the particles come to rest. It is named after William Henry Bragg, who discovered it in 1903.

Fast neutron therapy utilizes high energy neutrons typically between 50 and 70 MeV to treat cancer. Most fast neutron therapy beams are produced by reactors, cyclotrons (d+Be) and linear accelerators. Neutron therapy is currently available in Germany, Russia, South Africa and the United States. In the United States, one treatment center is operational, in Seattle, Washington. The Seattle center uses a cyclotron which produces a proton beam impinging upon a beryllium target.

In radiotherapy, radiation treatment planning (RTP) is the process in which a team consisting of radiation oncologists, radiation therapist, medical physicists and medical dosimetrists plan the appropriate external beam radiotherapy or internal brachytherapy treatment technique for a patient with cancer.
Electron therapy or electron beam therapy (EBT) is a kind of external beam radiotherapy where electrons are directed to a tumor site for medical treatment of cancer.

Tomotherapy is a type of radiation therapy treatment machine. In tomotherapy a thin radiation beam is modulated as it rotates around the patient, while they are moved through the bore of the machine. The name comes from the use of a strip-shaped beam, so that only one “slice” of the target is exposed at any one time by the radiation. The external appearance of the system and movement of the radiation source and patient can be considered analogous to a CT scanner, which uses lower doses of radiation for imaging. Like a conventional machine used for X-ray external beam radiotherapy, a linear accelerator generates the radiation beam, but the external appearance of the machine, the patient positioning, and treatment delivery is different. Conventional linacs do not work on a slice-by-slice basis but typically have a large area beam which can also be resized and modulated.
FLUKA is a fully integrated Monte Carlo simulation package for the interaction and transport of particles and nuclei in matter. FLUKA has many applications in particle physics, high energy experimental physics and engineering, shielding, detector and telescope design, cosmic ray studies, dosimetry, medical physics, radiobiology. A recent line of development concerns hadron therapy.

Cobalt therapy is the medical use of gamma rays from the radioisotope cobalt-60 to treat conditions such as cancer. Beginning in the 1950s, cobalt-60 was widely used in external beam radiotherapy (teletherapy) machines, which produced a beam of gamma rays which was directed into the patient's body to kill tumor tissue. Because these "cobalt machines" were expensive and required specialist support, they were often housed in cobalt units. Cobalt therapy was a revolutionary advance in radiotherapy in the post-World War II period but is now being replaced by other technologies such as linear accelerators.
Particle therapy is a form of external beam radiotherapy using beams of energetic neutrons, protons, or other heavier positive ions for cancer treatment. The most common type of particle therapy as of August 2021 is proton therapy.
Radiobiology is a field of clinical and basic medical sciences that involves the study of the effects of ionizing radiation on living things, in particular health effects of radiation. Ionizing radiation is generally harmful and potentially lethal to living things but can have health benefits in radiation therapy for the treatment of cancer and thyrotoxicosis. Its most common impact is the induction of cancer with a latent period of years or decades after exposure. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Controlled doses are used for medical imaging and radiotherapy.
Intraoperative electron radiation therapy is the application of electron radiation directly to the residual tumor or tumor bed during cancer surgery. Electron beams are useful for intraoperative radiation treatment because, depending on the electron energy, the dose falls off rapidly behind the target site, therefore sparing underlying healthy tissue.

The Harvard Cyclotron Laboratory operated from 1949 to 2002. It was most notable for its contributions to the development of proton therapy.
Pencil beam scanning is the practice of steering a beam of radiation or charged particles across an object. It is often used in proton therapy, to reduce unnecessary radiation exposure to surrounding non-cancerous cells.
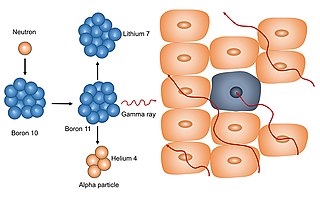
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.

The The Svedberg Laboratory (TSL) is a university facility, based in Uppsala, Sweden. The activities at TSL are based around the particle accelerator Gustaf Werner cyclotron.













