Eukaryotic translation is the biological process by which messenger RNA is translated into proteins in eukaryotes. It consists of four phases: initiation, elongation, termination, and recapping.

l-Kynurenine is a metabolite of the amino acid l-tryptophan used in the production of niacin.

Indoleamine-pyrrole 2,3-dioxygenase (IDO or INDO EC 1.13.11.52) is a heme-containing enzyme physiologically expressed in a number of tissues and cells, such as the small intestine, lungs, female genital tract or placenta. In humans is encoded by the IDO1 gene. IDO is involved in tryptophan metabolism. It is one of three enzymes that catalyze the first and rate-limiting step in the kynurenine pathway, the O2-dependent oxidation of L-tryptophan to N-formylkynurenine, the others being indolamine-2,3-dioxygenase 2 (IDO2) and tryptophan 2,3-dioxygenase (TDO). IDO is an important part of the immune system and plays a part in natural defense against various pathogens. It is produced by the cells in response to inflammation and has an immunosuppressive function because of its ability to limit T-cell function and engage mechanisms of immune tolerance. Emerging evidence suggests that IDO becomes activated during tumor development, helping malignant cells escape eradication by the immune system. Expression of IDO has been described in a number of types of cancer, such as acute myeloid leukemia, ovarian cancer or colorectal cancer. IDO is part of the malignant transformation process and plays a key role in suppressing the anti-tumor immune response in the body, so inhibiting it could increase the effect of chemotherapy as well as other immunotherapeutic protocols. Furthermore, there is data implicating a role for IDO1 in the modulation of vascular tone in conditions of inflammation via a novel pathway involving singlet oxygen.

Cluster of Differentiation 86 is a protein constitutively expressed on dendritic cells, Langerhans cells, macrophages, B-cells, and on other antigen-presenting cells. Along with CD80, CD86 provides costimulatory signals necessary for T cell activation and survival. Depending on the ligand bound, CD86 can signal for self-regulation and cell-cell association, or for attenuation of regulation and cell-cell disassociation.

Lankenau Institute for Medical Research (LIMR), founded in 1927, is a nonprofit, biomedical research institute located on the campus of Lankenau Medical Center in Wynnewood, Pennsylvania, serving as the research division of the Main Line Health System in suburban Philadelphia. LIMR focuses on studies of cancer, cardiovascular, autoimmune, gastrointestinal and other diseases. It houses a center for population health research.
In enzymology, an indole 2,3-dioxygenase (EC 1.13.11.17) is an enzyme that catalyzes the chemical reaction
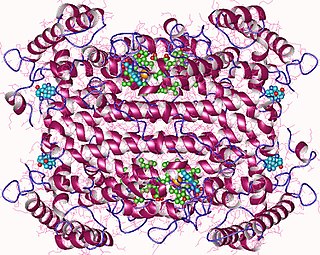
In enzymology, tryptophan 2,3-dioxygenase (EC 1.13.11.11) is a heme enzyme that catalyzes the oxidation of L-tryptophan (L-Trp) to N-formyl-L-kynurenine, as the first and rate-limiting step of the kynurenine pathway.

Tryptophan hydroxylase 2 (TPH2) is an isozyme of tryptophan hydroxylase found in vertebrates. In humans, TPH2 is primarily expressed in the serotonergic neurons of the brain, with the highest expression in the raphe nucleus of the midbrain. Until the discovery of TPH2 in 2003, serotonin levels in the central nervous system were believed to be regulated by serotonin synthesis in peripheral tissues, in which tryptophan hydroxylase is the dominant form.

Tryptophanyl-tRNA synthetase, cytoplasmic is an aminoacyl-tRNA synthetase enzyme that attaches the amino acid tryptophan to its cognate tRNA. In humans, it is encoded by the WARS gene.

Homeobox D10, also known as HOXD10, is a protein which in humans is encoded by the HOXD10 gene.
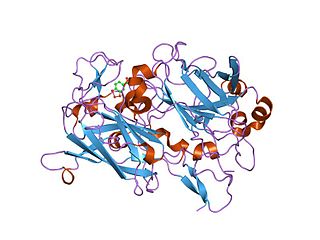
Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine triphosphate (ATP) generation to xenobiotic degradation, the use of dioxygen as a biological oxidant is widespread and varied in the exact mechanism of its use. Enzymes employ many different schemes to use dioxygen, and this largely depends on the substrate and reaction at hand.

Zinc finger and BTB domain-containing protein 32 is a protein that in humans is encoded by the 1960 bp ZBTB32 gene. The 52 kDa protein is a transcriptional repressor and the gene is expressed in T and B cells upon activation, but also significantly in testis cells. It is a member of the Poxviruses and Zinc-finger (POZ) and Krüppel (POK) family of proteins, and was identified in multiple screens involving either immune cell tumorigenesis or immune cell development.

Tryptophan hydroxylase 1 (TPH1) is an isoenzyme of tryptophan hydroxylase which in humans is encoded by the TPH1 gene.

Kynurenine 3-monooxygenase is an enzyme that in humans is encoded by the KMO gene.
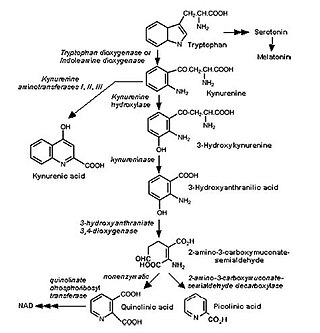
The kynurenine pathway is a metabolic pathway leading to the production of nicotinamide adenine dinucleotide (NAD+). Metabolites involved in the kynurenine pathway include tryptophan, kynurenine, kynurenic acid, xanthurenic acid, quinolinic acid, and 3-hydroxykynurenine. The kynurenine pathway is responsible for about 95% of total tryptophan catabolism. Disruption in the pathway is associated with certain genetic and psychiatric disorders.
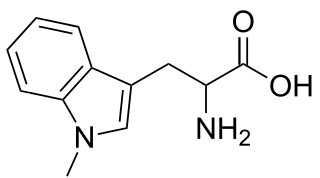
1-Methyltryptophan is a chemical compound that is an inhibitor of the tryptophan catabolic enzyme indoleamine 2,3-dioxygenase. It is a chiral compound that can exist as both D- and L-enantiomers.

George C. Prendergast is an American biomedical scientist. His research has focused on cancer pathobiology and immunology. Since 2004, he has been the President and CEO of Lankenau Institute for Medical Research, a cancer-focused research center in the U.S. He is also the co-director of the Program in Cancer Cell Biology & Signaling at the Sidney Kimmel Cancer Center, Thomas Jefferson University.

Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.

Epacadostat is an investigational drug for cancer. Epacadostat is an inhibitor of indoleamine 2,3-dioxygenase-1 (IDO1). Epacadostat inhibits IDO1 by competitively blocking it, without interfering with IDO2 or tryptophan 2,3-dioxygenase (TDO). It has antitumor activity in some models, though is most effective when combined with other immunotherapy agents.

Linrodostat is an experimental drug being studied for its immunomodulating and antineoplastic activities.





















