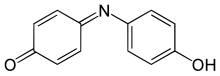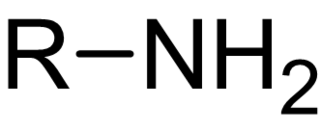
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Formally, amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include phenolic acids, flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.

Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.
Nascent hydrogen is an outdated concept in organic chemistry that was once invoked to explain dissolving-metal reactions, such as the Clemmensen reduction and the Bouveault–Blanc reduction. Since organic compounds do not react with H2, a special state of hydrogen was postulated. It is now understood that dissolving-metal reactions occur at the metal surface, and the concept of nascent hydrogen has been discredited in organic chemistry. However, the formation of atomic hydrogen is largely invoked in inorganic chemistry and corrosion sciences to explain hydrogen embrittlement in metals exposed to electrolysis and anaerobic corrosion (e.g., dissolution of zinc in strong acids (HCl) and aluminium in strong bases (NaOH)). The mechanism of hydrogen embrittlement was first proposed by Johnson (1875). The inability of hydrogen atoms to react with organic reagents in organic solvents does not exclude the transient formation of hydrogen atoms capable to immediately diffuse into the crystal lattice of common metals (steel, titanium) different from these of the platinoid group (Pt, Pd, Rh, Ru, Ni) which are well known to dissociate molecular dihydrogen (H2) into atomic hydrogen.

Devarda's alloy is an alloy of aluminium (44% – 46%), copper (49% – 51%) and zinc (4% – 6%).

In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
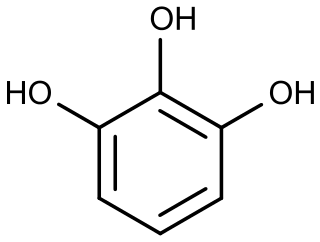
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a water-soluble, white solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomers of benzenetriols.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.

Rosocyanine and rubrocurcumin are two red colored materials, which are formed by the reaction between curcumin and borates.

2,6-Dichlorophenolindophenol is a chemical compound used as a redox dye. When oxidized, DCPIP is blue with a maximal absorption at 600 nm; when reduced, DCPIP is colorless.
Polyphenol oxidase, an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule.
Berthelot's reagent is an alkaline solution of phenol and hypochlorite, used in analytical chemistry. It is named after its inventor, Marcellin Berthelot. Ammonia reacts with Berthelot's reagent to form a blue product which is used in a colorimetric method for determining ammonia. The reagent can also be used for determining urea. In this case the enzyme urease is used to catalyze the hydrolysis of urea into carbon dioxide and ammonia. The ammonia is then determined with Berthelot's reagent.