Related Research Articles
Bavituximab (PGN401) is a human-mouse chimeric monoclonal antibody against phosphatidylserine, which is a component of cell membranes that is exposed when a cell is transformed into solid tumor cancer cell or dies, and when cells are infected with hepatitis C. The process of cell death is highly controlled and so there usually no immune response to phosphatidylserine but when bavituximab binds to it, the conjugate appears to stimulate an immune response in humans.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Milatuzumab is an anti-CD74 humanized monoclonal antibody for the treatment of multiple myeloma non-Hodgkin's lymphoma and chronic lymphocytic leukemia.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seagen in the US.
Glembatumumab vedotin is an antibody-drug conjugate (ADC) that targets cancer cells expressing transmembrane glycoprotein NMB (GPNMB).

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.
Amatuximab is a chimeric monoclonal antibody designed for the treatment of cancer. It was developed by Morphotek, Inc.
Demcizumab is a humanized monoclonal antibody which is used to treat patients with pancreatic cancer or non-small cell lung cancer. Demcizumab has completed phase 1 trials and is currently undergoing phase 2 trials. Demcizumab was developed by OncoMed Pharmaceuticals in collaboration with Celgene.
Lirilumab (INN) is a human monoclonal antibody designed for the treatment of cancer. It binds to KIR2DL1/2/3.

Vadastuximab talirine is an antibody-drug conjugate (ADC) directed to CD33 (siglec-3) which is a transmembrane receptor expressed on cells of myeloid lineage. The experimental drug, being developed by Seattle Genetics, was in clinical trials for the treatment of acute myeloid leukemia (AML).
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
ImmunoGen, Inc. was a biotechnology company focused on the development of antibody-drug conjugate (ADC) therapeutics for the treatment of cancer. ImmunoGen was founded in 1981 and was headquartered in Waltham, Massachusetts.
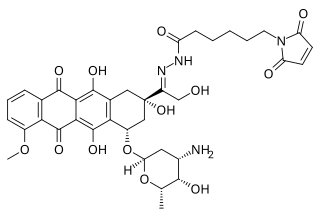
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid-sensitive linker.
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.
Denintuzumab mafodotin is a humanized monoclonal antibody-drug conjugate designed for the treatment of CD19-positive acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma. It consists of an anti-CD19 mAb linked to monomethyl auristatin F (MMAF), a cytotoxic agent. This drug was developed by Seattle Genetics.
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.
Rovalpituzumab tesirine (Rova-T) is an experimental antibody-drug conjugate targeting the protein DLL3 on tumor cells. It was originally developed by Stemcentrx and was purchased by AbbVie. It was tested for use in small-cell lung cancer, but development was terminated after unsuccessful phase III trial.

Camidanlumab tesirine is an antibody-drug conjugate (ADC) composed of a human antibody that binds to the protein CD25, conjugated to a pyrrolobenzodiazepine dimer toxin. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell Hodgkin's lymphoma (HL) and non-Hodgkin lymphoma (NHL), and for the treatment of B-cell acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).
Tisotumab vedotin, sold under the brand name Tivdak, is an antibody-drug conjugate used to treat cervical cancer. It is a combination of tisotumab, a monoclonal antibody against tissue factor, and monomethyl auristatin E (MMAE), a potent inhibitor of cell division. It is administered by infusion into a vein.
References
- 1 2 Clinical trial number NCT02202785 for "A Study of MLN0264 in Patients With Pancreatic Cancer" at ClinicalTrials.gov
- 1 2 Clinical trial number NCT02202759 for "A Study of MLN0264 in Patients With Cancer of the Stomach or Gastroesophageal Junction" at ClinicalTrials.gov
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended INN: List 74" (PDF). WHO Drug Information. 29 (3): 402–3. 2015.
- ↑ Almhanna K, Wright D, Mercade TM, et al. (2017). "A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C". Invest New Drugs. 35 (5): 634–641. doi:10.1007/s10637-017-0473-9. PMID 28527133. S2CID 3653513.