
A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.
Calcicludine (CaC) is a protein toxin from the venom of the green mamba that inhibits high-voltage-activated calcium channels, especially L-type calcium channels.

Calciseptine (CaS) is a natural neurotoxin isolated from the black mamba Dendroaspis p. polylepis venom. This toxin consists of 60 amino acids with four disulfide bonds. Calciseptine specifically blocks L-type calcium channels, but not other voltage-dependent Ca2+ channels such as N-type and T-type channels.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channel's initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.

Agatoxins are a class of chemically diverse polyamine and peptide toxins which are isolated from the venom of various spiders. Their mechanism of action includes blockade of glutamate-gated ion channels, voltage-gated sodium channels, or voltage-dependent calcium channels. Agatoxin is named after the funnel web spider which produces a venom containing several agatoxins. There are different agatoxins. The ω-agatoxins are approximately 100 amino acids in length and are antagonists of voltage-sensitive calcium channels and also block the release of neurotransmitters. For instance, the ω-agatoxin 1A is a selective blocker and will block L-type calcium channels whereas the ω-agatoxin 4B will inhibit voltage sensitive P-type calcium channels. The μ-agatoxins only act on insect voltage-gated sodium channels.
Imperatoxin I (IpTx) is a peptide toxin derived from the venom of the African scorpion Pandinus imperator.
BmKAEP is a neurotoxin from the venom of the Manchurian scorpion (Mesobuthus martensii). It is a β-toxin, which shift the activation voltage of sodium channels towards more negative potentials.
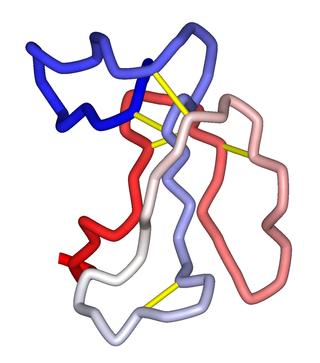
α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.
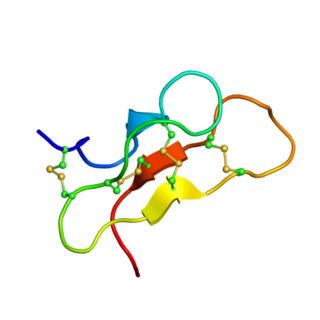
delta-Palutoxins (δ-palutoxins) consist of a homologous group of four insect-specific toxins from the venom of the spider Pireneitega luctuosa. They show a high toxicity against Spodoptera litura larvae by inhibiting sodium channels, leading to strong paralytic activity and eventually to the death of the insect.
Huwentoxins (HWTX) are a group of neurotoxic peptides found in the venom of the Chinese bird spider Haplopelma schmidti. The species was formerly known as Haplopelma huwenum, Ornithoctonus huwena and Selenocosmia huwena. While structural similarity can be found among several of these toxins, HWTX as a group possess high functional diversity.
CSTX is a name given to a group of closely related neurotoxic peptides present in the venom of the wandering spider Cupiennius salei. There are twenty types so far described for this protein group. However, some are reclassified into cupiennins group of toxin, including CSTX-3, -4, -5, and -6, because of their chemical affinity. The first thirteen were isolated and identified in 1994 by Lucia Kuhn-Nentwig, Johann Schaller, and Wolfgang Nentwig of the Zoological Institute at the University of Bern, Switzerland. The different types are most likely the products of splicing variant of the same gene. They are all L-type calcium channel blockers, and also exhibit cytolytic activity by forming an alpha-helix across the cell membrane in mammalian neurons. They also inhibit voltage-gated calcium channels in insect neurons.

Oxyopes lineatus is a species of spider in the family Oxyopidae, the so-called lynx spiders.
Oxotoxins, or oxytoxins, are a group of neurotoxins present in the venom of lynx spiders belonging to the genus Oxyopes, hence the name oxytoxin. They are disulfide-rich peptides. Only two types are so far reported from two different species, the larger oxytoxin 1 (OxyTx1) from Oxyopes kitabensis, and the smaller oxytoxin 2 (OxyTx2) from Oxyopes lineatus. OxyTx1, the first known oxytoxin, was discovered in 2002. It was found to enhance the lethal efficacy of the spider venom by acting together with oxyopinins. It is composed of 69 amino acid residue, which are cross-linked by five disulfide bridges. It is a large peptide having a molecular mass of 8059.2 Da; but shows the size of 9,109.4 Da due to the presence of disulfide bridges. It is a potent insecticide, but non-toxic to mice up to 1 μg/20-g mouse. It acts synergistically with oxyopinins of the same venom to increase the insecticidal effect.
Agelenin, also called U1-agatoxin-Aop1a, is an antagonist of the presynaptic P-type calcium channel in insects. This neurotoxic peptide consists of 35 amino acids and can be isolated from the venom of the spider Allagelena opulenta.
AmmTX3, produced by Androctonus mauretanicus, is a scorpion toxin of the α-KTX15 subfamily. The toxin is known for its ability to act as a specific Kv4 channel blocker, and thereby reducing the A-type potassium current through this channel.
HsTx1 is a toxin from the venom of the scorpion Heterometrus spinifer. HsTx1 is a very potent inhibitor of the rat Kv1.3 voltage-gated potassium channel.

Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).
Beta-mammal toxin Cn2, also known as Cn2 toxin, is a single chain β-scorpion neurotoxic peptide and the primary toxin in the venom of the Centruroides noxius Hoffmann scorpion. The toxin specifically targets mammalian Nav1.6 voltage-gated sodium channels (VGSC).
Ptu1 is a toxin that can reversibly bind N-type calcium channels. Its isolated from the assassin bug Peirates turpis. The toxin belongs to the inhibitory cystine knot structural family (ICK) that has a core of disulfide bonds with four loops emerging from it.
AsKC11 is a toxin found in the venom of the sea anemone, Anemonia sulcata. This toxin is part of the Kunitz peptide family and has been shown to be an activator of G protein-coupled inwardly-rectifying potassium (GIRK) channels 1/2, involved in the regulation of cellular excitability.







