Related Research Articles
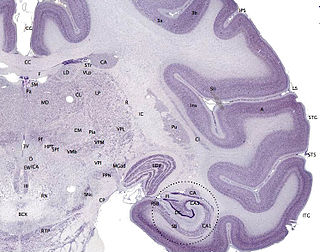
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness.
The development of the nervous system, or neural development, or neurodevelopment, refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.
In vertebrates, a neuroblast or primitive nerve cell is a postmitotic cell that does not divide further, and which will develop into a neuron after a migration phase. In invertebrates such as Drosophila, neuroblasts are neural progenitor cells which divide asymmetrically to produce a neuroblast, and a daughter cell of varying potency depending on the type of neuroblast. Vertebrate neuroblasts differentiate from radial glial cells and are committed to becoming neurons. Neural stem cells, which only divide symmetrically to produce more neural stem cells, transition gradually into radial glial cells. Radial glial cells, also called radial glial progenitor cells, divide asymmetrically to produce a neuroblast and another radial glial cell that will re-enter the cell cycle.
Neuroepithelial cells, or neuroectodermal cells, form the wall of the closed neural tube in early embryonic development. The neuroepithelial cells span the thickness of the tube's wall, connecting with the pial surface and with the ventricular or lumenal surface. They are joined at the lumen of the tube by junctional complexes, where they form a pseudostratified layer of epithelium called neuroepithelium.

A progenitor cell is a biological cell that, like a stem cell, has a tendency to differentiate into a specific type of cell, but is already more specific than a stem cell and is pushed to differentiate into its "target" cell. The most important difference between stem cells and progenitor cells is that stem cells can replicate indefinitely, whereas progenitor cells can divide only a limited number of times. Controversy about the exact definition remains and the concept is still evolving.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).
An asymmetric cell division produces two daughter cells with different cellular fates. This is in contrast to symmetric cell divisions which give rise to daughter cells of equivalent fates. Notably, stem cells divide asymmetrically to give rise to two distinct daughter cells: one copy of the original stem cell as well as a second daughter programmed to differentiate into a non-stem cell fate.

Homeobox protein EMX1 is a protein that in humans is encoded by the EMX1 gene. The transcribed EMX1 gene is a member of the EMX family of transcription factors. The EMX1 gene, along with its family members, are expressed in the developing cerebrum. Emx1 plays a role in specification of positional identity, the proliferation of neural stem cells, differentiation of layer-specific neuronal phenotypes and commitment to a neuronal or glial cell fate.

The ganglionic eminence (GE) is a transitory structure in the development of the nervous system that guides cell and axon migration. It is present in the embryonic and fetal stages of neural development found between the thalamus and caudate nucleus.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.
The Protomap is a primordial molecular map of the functional areas of the mammalian cerebral cortex during early embryonic development, at a stage when neural stem cells are still the dominant cell type. The protomap is a feature of the ventricular zone, which contains the principal cortical progenitor cells, known as radial glial cells. Through a process called 'cortical patterning', the protomap is patterned by a system of signaling centers in the embryo, which provide positional information and cell fate instructions. These early genetic instructions set in motion a development and maturation process that gives rise to the mature functional areas of the cortex, for example the visual, somatosensory, and motor areas. The term protomap was coined by Pasko Rakic. The protomap hypothesis was opposed by the protocortex hypothesis, which proposes that cortical proto-areas initially have the same potential, and that regionalization in large part is controlled by external influences, such as axonal inputs from the thalamus to the cortex. However, a series of papers in the year 2000 and in 2001 provided strong evidence against the protocortex hypothesis, and the protomap hypothesis has been well accepted since then. The protomap hypothesis, together with the related radial unit hypothesis, forms our core understanding of the embryonic development of the cerebral cortex. Once the basic structure is present and cortical neurons have migrated to their final destinations, many other processes contribute to the maturation of functional cortical circuits.

Ganglion mother cells (GMCs) are cells involved in neurogenesis, in non-mammals, that divide only once to give rise to two neurons, or one neuron and one glial cell or two glial cells, and are present only in the central nervous system. They are also responsible for transcription factor expression. While each ganglion mother cell necessarily gives rise to two neurons, a neuroblast can asymmetrically divide multiple times. GMCs are the progeny of type I neuroblasts. Neuroblasts asymmetrically divide during embryogenesis to create GMCs. GMCs are only present in certain species and only during the embryonic and larval stages of life. Recent research has shown that there is an intermediate stage between a GMC and two neurons. The GMC forms two ganglion cells which then develop into neurons or glial cells. Embryonic neurogenesis has been extensively studied in Drosophila melanogaster embryos and larvae.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.

Corticogenesis is the process in which the cerebral cortex of the brain is formed during the development of the nervous system. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.

A cerebral organoid, or brain organoid, describes an artificially grown, in vitro, miniature organ resembling the brain. Cerebral organoids are created by culturing pluripotent stem cells in a three-dimensional rotational bioreactor, and they develop over a course of months. The brain is an extremely complex system of heterogeneous tissues and consists of a diverse array of neurons. This complexity has made studying the brain and understanding how it works a difficult task in neuroscience, especially when it comes to neurodegenerative diseases. The purpose of creating an in vitro neurological model is to study these diseases in a more simple and variable space. This 3D model is free of many potential in vivo limitations. The varying physiology between human and other mammalian models limits the scope of study in neurological disorders. Cerebral organoids are synthesized tissues that contain several types of nerve cells and have anatomical features that recapitulate regions of the cortex observed in brains. Cerebral organoids are most similar to layers of neurons called the cortex and choroid plexus. In some cases, structures similar to the retina, meninges and hippocampus can form. Stem cells have the potential to grow into many different types of tissues, and their fate is dependent on many factors. Below is an image showing some of the chemical factors that can lead stem cells to differentiate into various neural tissues; a more in-depth table of generating specific organoid identity has been published since. Similar techniques are used on stem cells used to grow cerebral organoids.
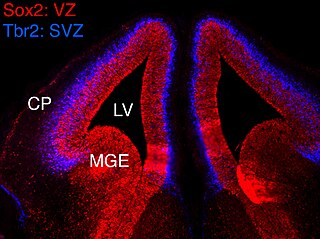
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). It occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.

The Radial Unit Hypothesis (RUH) is a conceptual theory of cerebral cortex development, first described by Pasko Rakic. The RUH states that the cerebral cortex develops during embryogenesis as an array of interacting cortical columns, or 'radial units', each of which originates from a transient stem cell layer called the ventricular zone, which contains neural stem cells known as radial glial cells.
Arnold Richard Kriegstein is a neurologist and neuroscientist who is the John Bowes Distinguished Professor in Stem Cell and Tissue Biology at the University of California, San Francisco where he serves as director of the UCSF Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research. His main research interests include neural stem cell and brain development. He is a member of the National Academy of Medicine.
References
- 1 2 3 Kowalczyk, Tom; Pontious, Adria; Englund, Chris; Daza, Ray A. M.; Bedogni, Francesco; Hodge, Rebecca; Attardo, Alessio; Bell, Chris; Huttner, Wieland B. (2009-10-01). "Intermediate Neuronal Progenitors (Basal Progenitors) Produce Pyramidal–Projection Neurons for All Layers of Cerebral Cortex". Cerebral Cortex. 19 (10): 2439–2450. doi:10.1093/cercor/bhn260. ISSN 1047-3211. PMC 2742596 . PMID 19168665.
- 1 2 3 Harris, Lachlan; Zalucki, Oressia; Gobius, Ilan; McDonald, Hannah; Osinki, Jason; Harvey, Tracey J.; Essebier, Alexandra; Vidovic, Diana; Gladwyn-Ng, Ivan; Burne, Thomas H.; Heng, Julian I.; Richards, Linda J.; Gronostajski, Richard M.; Piper, Michael (2016-10-28). "Transcriptional Regulation of Intermediate Progenitor Cell Generation during Hippocampal Development". Development. 143: 4620–4630. doi: 10.1242/dev.140681 .
- 1 2 Martínez-Cerdeño, Verónica; Noctor, Stephen C.; Kriegstein, Arnold R. (July 2006). "The Role of Intermediate Progenitor Cells in the Evolutionary Expansion of the Cerebral Cortex". Cereb Cortex. 30 (Supplement 1): 152–161. doi: 10.1093/cercor/bhk017 . PMID 16766701.
- ↑ Pontious, Adria; Kowalczyk, Tom; Englund, Chris; Hevner, Robert F. (2008). "Role of Intermediate Progenitor Cells in Cerebral Cortex Development". Developmental Neuroscience. 30 (1–3): 24–32. doi:10.1159/000109848. ISSN 0378-5866. PMID 18075251.
- 1 2 3 Noctor, Stephen C.; Martínez-Cerdeño, Verónica; Kriegstein, Arnold R. (May 2007). "Contribution of Intermediate Progenitor Cells to Cortical Histogenesis". Arch Neurol. 64 (5): 639–642. doi: 10.1001/archneur.64.5.639 . PMID 17502462.
- 1 2 Javaherian, Ashkan; Kriegstein, Arnold (July 2009). "A Stem Cell Niche for Intermediate Progenitor Cells of the Embryonic Cortex". Cereb Cortex. 19: 70–77. doi: 10.1093/cercor/bhp029 . PMC 2693531 . PMID 19346271.