
Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge, a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria. It is a potent functional analog of capsaicin, the active ingredient in chili peppers.

Euphorbia resinifera, the resin spurge, is a species of spurge native to Morocco, where it occurs on the slopes of the Atlas Mountains. The dried latex of the plant was used in ancient medicine. It contains resiniferatoxin, an extremely potent capsaicin analog tested as an analgesic since 1997.

WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
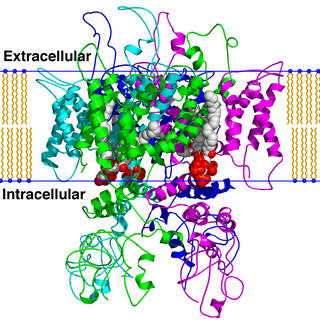
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Capsazepine is a synthetic antagonist of capsaicin. It is used as a biochemical tool in the study of TRPV ion channels.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid. AM404 is one of the AM cannabinoids discovered by Alexandros Makriyannis and his team.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.
GSK-189,254 is a potent and selective H3 histamine receptor inverse agonist developed by GlaxoSmithKline. It has subnanomolar affinity for the H3 receptor (Ki = 0.2nM) and selectivity of over 10,000x for H3 over other histamine receptor subtypes. Animal studies have shown it to possess not only stimulant and nootropic effects, but also analgesic action suggesting a role for H3 receptors in pain processing in the spinal cord. GSK-189,254 and several other related drugs are currently being investigated as a treatment for Alzheimer's disease and other forms of dementia, as well as possible use in the treatment of conditions such as narcolepsy, or neuropathic pain which do not respond well to conventional analgesic drugs.
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.

LASSBio-881 is a drug which acts as both a non-selective partial agonist of the CB1 and CB2 cannabinoid receptors, and also as an antagonist of the TRPV1 receptor, as well as having antioxidant effects. It has potent anti-inflammatory and anti-hyperalgesic effects in animal studies.

Vanillotoxins are neurotoxins found in the venom of the tarantula Psalmopoeus cambridgei. They act as agonists for the transient receptor potential cation channel subfamily V member 1 (TRPV1), activating the pain sensory system. VaTx1 and 2 also act as antagonists for the Kv2-type voltage-gated potassium channel (Kv2), inducing paralytic behavior in small animals.

The vanilloids are compounds which possess a vanillyl group. They include vanillyl alcohol, vanillin, vanillic acid, acetovanillon, vanillylmandelic acid, homovanillic acid, capsaicin, etc. Isomers are the isovanilloids.

Phenylacetylrinvanil (IDN-5890) is a synthetic analogue of capsaicin which acts as a potent and selective agonist for the TRPV1 receptor, with slightly lower potency than resiniferatoxin, though still around 300 times the potency of capsaicin. It is an amide of vanillylamine and ricinoleic acid, with the hydroxyl group on ricinoleic acid esterified with phenylacetic acid. It is used to study the function of the TRPV1 receptor and its downstream actions, and has also shown anti-cancer effects in vitro.

AMG-9810 is a drug which acts as a potent and selective antagonist for the TRPV1 receptor. It has analgesic and antiinflammatory effects and is used in scientific research, but has not been developed for medical use. It has high antagonist potency and good bioavailability and pharmacokinetics, and so has been used to study the role of TRPV1 in areas other than pain perception, such as its roles in the brain.

AMG-517 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It was developed as a potential treatment for chronic pain, but while it was an effective analgesic in animal studies it was dropped from human clinical trials at Phase I due to producing hyperthermia as a side effect, as well as poor water solubility. It is still used in scientific research into the function of the TRPV1 channel and its role in pain and inflammation, and has been used as a template for the design of several newer analogues which have improved properties.

SB-705498 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It has been evaluated in clinical trials for the treatment of rhinitis and chronic cough.
















