Related Research Articles

Digital microfluidics (DMF) is a platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.

Cyclic voltammetry (CV) is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography - MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides structural identity of the individual components with high molecular specificity and detection sensitivity. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC-MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries.
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
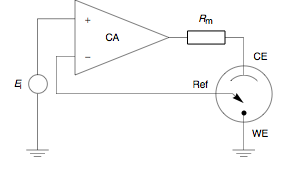
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A Bipotentiostat and polypotentiostat are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram which plots the current produced by the analyte versus the potential of the working electrode.

The Orbiting Frog Otolith (OFO) was a NASA space program which sent two bullfrogs into orbit on 9 November 1970 for the study of weightlessness. The name, derived through common use, was a functional description of the biological experiment carried by the satellite. Otolith referred to the frog's inner ear balance mechanism.
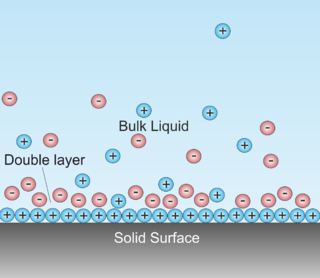
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".

Linear sweep voltammetry is a voltammetric method where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time. Oxidation or reduction of species is registered as a peak or trough in the current signal at the potential at which the species begins to be oxidized or reduced.
Squarewave voltammetry (SWV) is a form of linear potential sweep voltammetry that uses a combined square wave and staircase potential applied to a stationary electrode. It has found numerous applications in various fields, including within medicinal and various sensing communities.
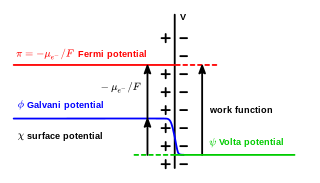
In electrochemistry, the Galvani potential is the electric potential difference between two points in the bulk of two phases. These phases can be two different solids, or a solid and a liquid.
A rotating ring-disk electrode (RRDE) is a double working electrode used in hydrodynamic voltammetry, very similar to a rotating disk electrode (RDE). The electrode rotates during experiments inducing a flux of analyte to the electrode. This system used in electrochemical studies when investigating reaction mechanisms related to redox chemistry and other chemical phenomena.
Hydrodynamic voltammetry is a form of voltammetry in which the analyte solution flows relative to a working electrode. In many voltammetry techniques, the solution is intentionally left still to allow diffusion controlled mass transfer. When a solution is made to flow, through stirring or some other physical mechanism, it is very important to the technique to achieve a very controlled flux or mass transfer in order to obtain predictable results. These methods are types of electrochemical studies which use potentiostats to investigate reaction mechanisms related to redox chemistry among other chemical phenomenon.
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employs a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential (volts) and current (amps) is monitored over time (seconds). In a properly run experiment an analyte is quantitatively converted from its original oxidation state to a new oxidation state, either reduced or oxidized. As the substrate is consumed, the current also decreases, approaching zero when the conversion nears completion.
In electrochemistry, polarization is a collective term for certain mechanical side-effects by which isolating barriers develop at the interface between electrode and electrolyte. These side-effects influence the reaction mechanisms, as well as the chemical kinetics of corrosion and metal deposition. In a reaction we can displace the bonding electrons by attacking reagents. The electronic displacement in turn may be due to certain effects, some of which are permanent, and the others are temporary. Those effects which are permanently operating in the molecule are known as polarization effects, and those effects which are brought into play by attacking reagent are known as polarisability effects.
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the German electrochemist Heinz Gerischer. The interest in this domain is high in the context of development of renewable energy conversion and storage technology.
Scanning electrochemical microscopy (SECM) is a technique within the broader class of scanning probe microscopy (SPM) that is used to measure the local electrochemical behavior of liquid/solid, liquid/gas and liquid/liquid interfaces. Initial characterization of the technique was credited to University of Texas electrochemist, Allen J. Bard, in 1989. Since then, the theoretical underpinnings have matured to allow widespread use of the technique in chemistry, biology and materials science. Spatially resolved electrochemical signals can be acquired by measuring the current at an ultramicroelectrode (UME) tip as a function of precise tip position over a substrate region of interest. Interpretation of the SECM signal is based on the concept of diffusion-limited current. Two-dimensional raster scan information can be compiled to generate images of surface reactivity and chemical kinetics.
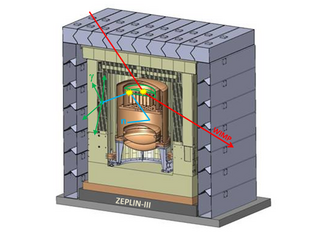
The ZEPLIN-III dark matter experiment attempted to detect galactic WIMPs using a 12 kg liquid xenon target. It operated at the Boulby Underground Laboratory in the period 2006–2011. This was the last in a series of xenon-based experiments in the ZEPLIN programme pursued originally by the UK Dark Matter Collaboration (UKDMC). The ZEPLIN-III project was led by Imperial College London and also included the Rutherford Appleton Laboratory and the University of Edinburgh in the UK, as well as LIP-Coimbra in Portugal and ITEP-Moscow in Russia. It ruled out cross-sections for elastic scattering of WIMPs off nucleons above 3.9 × 10−8 pb from the two science runs conducted at Boulby.
Ultraviolet-visible (UV-Vis) absorption spectroelectrochemistry (SEC) is a multiresponse technique that analyzes the evolution of the absorption spectra in UV-Vis regions during an electrode process. This technique provides information from an electrochemical and spectroscopic point of view. In this way, it enables a better perception about the chemical system of interest. On one hand, molecular information related to the electronic levels of the molecules is obtained from the evolution of the spectra. On the other hand, kinetic and thermodynamic information of the processes is obtained from the electrochemical signal.
Raman spectroelectrochemistry (Raman-SEC) is a technique that studies the inelastic scattering or Raman scattering of monochromatic light related to chemical compounds involved in an electrode process. This technique provides information about vibrational energy transitions of molecules, using a monochromatic light source, usually from a laser that belongs to the UV, Vis or NIR region. Raman spectroelectrochemistry provides specific information about structural changes, composition and orientation of the molecules on the electrode surface involved in an electrochemical reaction, being the Raman spectra registered a real fingerprint of the compounds.
References
- ↑ Potentiostats - Their Care and Feeding Archived 2006-06-13 at the Wayback Machine