
Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising six peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. The target molecules can be natural products, medicinally important active ingredients, or organic compounds of theoretical interest. Often the aim is to discover new route of synthesis for a target molecule for which there already exist known routes. Sometimes no route exists and the chemist wishes to find a viable route for the first time. One important purpose of total synthesis is the discovery of new chemical reactions and new chemical reagents.
Cyclopropene is an organic compound with the formula C3H4. It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit.
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
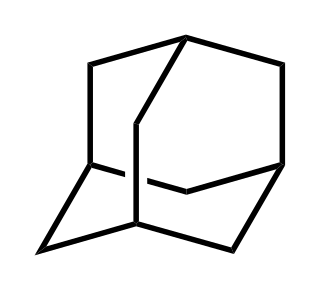
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consists of three connected cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C10H16, which include the somewhat similar twistane. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule.

In-Methylcyclophanes are organic compounds and members of a larger family of cyclophanes. These compounds are used to study how chemical bonds in molecules adapt to strain. In-methylcyclophanes in particular have a methyl group in proximity to a benzene ring. This is only possible when both methyl group and ring are attached to the same rigid scaffold. In one In-methylcyclophane molecule this is accomplished with a triptycene frame.

Triptycene is an aromatic hydrocarbon, the simplest iptycene molecule with the formula C2H2(C6H4)3. It is a white solid that is soluble in organic solvents. The compound has a paddle-wheel configuration with D3h symmetry. It is named after the medieval three-piece art panel, the triptych. Several substituted triptycenes are known. Barrelenes are structurally related. Due to the rigid framework and three-dimensional geometry, derivatives of triptycene have been well researched.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Synthetic molecular motors are molecular machines capable of continuous directional rotation under an energy input. Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion, some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors.

tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon acids, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.

Hexacene is an aromatic molecule consisting of six linearly-fused benzene rings. It is one of a series of linear polycyclic molecules created by such aromatic ring fusions, a series termed acenes—the previous in the series being pentacene and the next being heptacene. The CAS registry number for hexacene is 258-38-8, and it has a molecular weight of 328 g/mol. It and other acenes and their derivatives have been investigated in potential applications related to organic semiconductors.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with light or water vapor but is otherwise stable in storage. Xenon difluoride is a dense, white crystalline solid.
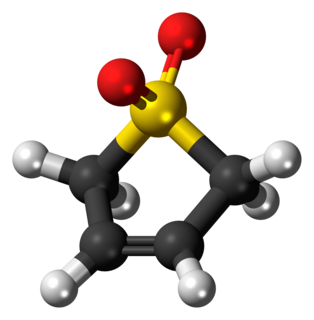
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.

Richard Frederick Heck was an American chemist noted for the discovery and development of the Heck reaction, which uses palladium to catalyze organic chemical reactions that couple aryl halides with alkenes. The analgesic naproxen is an example of a compound that is prepared industrially using the Heck reaction.
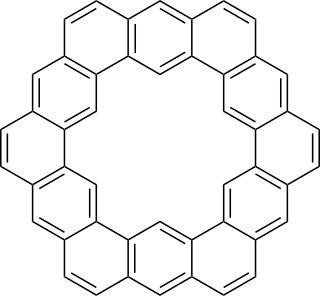
Kekulene is a polycyclic aromatic hydrocarbon and a circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.

Zethrene (dibenzo[de,mn]naphthacene) is a polycyclic aromatic hydrocarbon consisting of two phenalene units fused together. According to Clar's rule, the two exterior naphthalene units are truly aromatic and the two central double bonds are not aromatic at all. For this reason the compound is of some interest to academic research. Zethrene has a deep-red color and it is light sensitive - complete decomposition under a sunlight lamp occurs within 12 hours. The melting point is 262 °C.

Triangulene (also known as Clar's Hydrocarbon) is the smallest triplet-ground-state polybenzenoid. It exists as a diradical with the chemical formula C22H12. It was first hypothesized by Czech chemist Erich Clar in 1953. Its first confirmed synthesis was published in a February 2017 issue of Nature Nanotechnology, in a project led by researchers David Fox and Anish Mistry at the University of Warwick in collaboration with IBM. Other attempts by Japanese researchers have been successful only in making substituted triangulene derivatives.

1,2,4,5-Tetrabromobenzene is a fourfold symmetrically bromine-substituted benzene and starting material for liquid crystals and OLED materials, as well as for mono- and bis-aryines. 1,2,4,5-Tetrabromobenzene is an important metabolite of the completely brominated hexabromobenzene used as a flame retardant in the animal organism with liver-damaging properties.















