
Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.
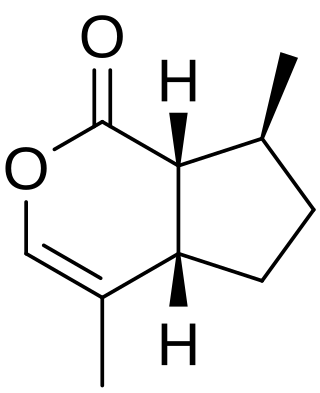
Nepetalactone is a name for multiple iridoid analog stereoisomers. Nepetalactones are produced by Nepeta cataria (catnip) and many other plants belonging to the genus Nepeta, in which they protect these plants from herbivorous insects by functioning as insect repellents. They are also produced by many aphids, in which they are sex pheromones. Nepetalactones are cat attractants, and cause the behavioral effects that catnip induces in domestic cats. However, they affect visibly only about two thirds of adult cats. They produce similar behavioral effects in many other felids, especially in lions and jaguars. In 1941, the research group of Samuel M. McElvain was the first to determine the structures of nepetalactones and several related compounds.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
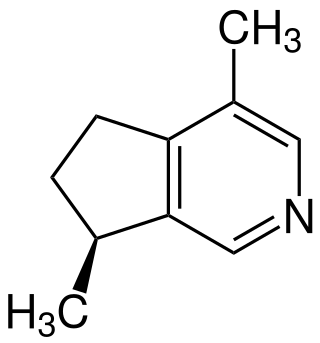
Actinidine is an iridoid produced in nature by a wide variety of plants and animals. It was the first cyclopentanoid monoterpene alkaloid to be discovered. It is one of several compounds that may be extracted from the valerian root and silver vine, as well as several types of insects in the larval and imaginal stages. Actinidine is a cat attractant, with effects like those of nepetalactone, the active compound found in catnip.
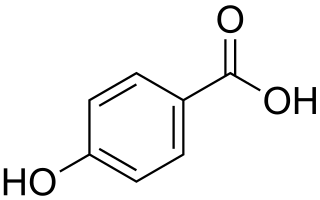
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
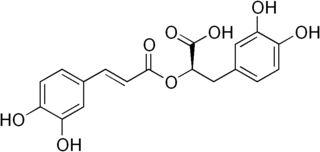
Rosmarinic acid, named after rosemary, is a polyphenol constituent of many culinary herbs, including rosemary, perilla, sage, mint, and basil.

Amino acid biosynthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids.

Iridoids are a type of monoterpenoids in the general form of cyclopentanopyran, found in a wide variety of plants and some animals. They are biosynthetically derived from 8-oxogeranial. Iridoids are typically found in plants as glycosides, most often bound to glucose.
In enzymology, an ent-copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction:

Nepeta cataria, commonly known as catnip and catmint, is a species of the genus Nepeta in the mint family, native to southern and eastern Europe, the Middle East, and Central Asia. It is widely naturalized in northern Europe, New Zealand, and North America. The common name catmint can also refer to the genus as a whole.

Catalpol is an iridoid glucoside. This natural product falls in the class of iridoid glycosides, which are simply monoterpenes with a glucose molecule attached.

Capsidiol is a terpenoid compound that accumulates in tobacco Nicotiana tabacum and chili pepper Capsicum annuum in response to fungal infection. Capsidiol is categorized under the broad term of phytoalexin, a class of low molecular weight plant secondary metabolites that are produced during infection. Phytoalexins are also characterized as a part of a two pronged response to infection which involves a short term response consisting of production of free radicals near the site of infection and a long term response involving the production of hormones and an increase in enzymes to biosynthesize phytoalexins such as capsidiol.

Aucubin is an iridoid glycoside. Iridoids are commonly found in plants and function as defensive compounds. Iridoids decrease the growth rates of many generalist herbivores.

Secologanin is a secoiridoid monoterpene synthesized from geranyl pyrophosphate in the mevalonate pathway. Secologanin then proceeds with dopamine or tryptamine to form ipecac and terpene indole alkaloids, respectively.

8-Oxogeranial is a chemical substance, that is a monoterpene. The terpenoid is produced by 8-hydroxygeraniol dehydrogenase which uses 8-hydroxygeraniol as its substrate. 8-Oxogeranial is itself a substrate for iridoid synthase which synthesizes cis–trans-iridodial and cis–trans-nepetalactol.

Nepetalactol is an iridoid. It is produced from 8-oxogeranial by the enzyme iridoid synthase. Nepetalactol is a substrate for the enzyme iridoid oxidase (IO) which produces 7-deoxyloganetic acid. It has been identified in Actinidia polygama as a major cat attractant, and a mosquito repellent. The fact that mosquitos bite cats with nepetalactol on their fur less often may explain why cats are attracted to silver vine in the first place.

7-Deoxyloganic acid is an iridoid monoterpene. 7-Deoxyloganic acid is produced from 7-deoxyloganetic acid by the enzyme 7-deoxyloganetic acid glucosyltransferase (7-DLGT). The metabolite is a substrate for the enzyme 7-deoxyloganic acid hydroxylase (7-DLH) which synthesizes loganic acid.
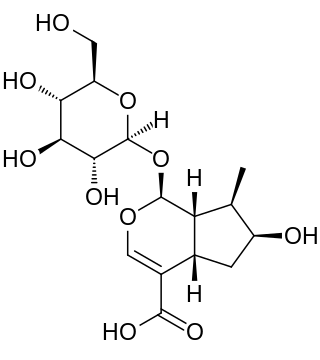
Loganic acid is an iridoid. Loganic acid is synthesized from 7-deoxyloganic acid by the enzyme 7-deoxyloganic acid hydroxylase (7-DLH). It is a substrate for the enzyme loganate O-methyltransferase for the production of loganin.

Harpagoside is a natural product found in the plant Harpagophytum procumbens, also known as devil's claw. It is the active chemical constituent responsible for the medicinal properties of the plant, which have been used for centuries by the Khoisan people of southern Africa to treat diverse health disorders, including fever, diabetes, hypertension, and various blood related diseases.
Sarah E. O'Connor is an American natural product chemist working to understand the molecular machinery involved in assembling important plant natural products – vinblastine, morphine, iridoids, secologanin – and how changing the enzymes involved in this pathway lead to diverse analogs. She was a Project Leader at the John Innes Centre in the UK between 2011 and 2019. O'Connor was appointed by the Max Planck Society in 2018 to head the Department of Natural Product Biosynthesis at the Max Planck Institute for Chemical Ecology in Jena, Germany, taking up her role during 2019.



















