Alkaloids are a class of naturally occurring organic compounds that mostly contain basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.

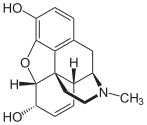
Morphine is a pain medication of the opiate family which is found naturally in a number of plants and animals, including humans. It acts directly on the central nervous system (CNS) to decrease the feeling of pain. It can be taken for both acute pain and chronic pain. It is frequently used for pain from myocardial infarction and during labor. It can be given by mouth, by injection into a muscle, by injection under the skin, intravenously, injection into the space around the spinal cord, or rectally. Maximum effect is reached after about 20 minutes when given intravenously and after 60 minutes when given by mouth, while duration of effect is 3–7 hours. Long-acting formulations also exist.

Opium is dried latex obtained from the seed capsules of the opium poppy Papaver somniferum. Approximately 12 percent of opium is made up of the analgesic alkaloid morphine, which is processed chemically to produce heroin and other synthetic opioids for medicinal use and for illegal drug trade. The latex also contains the closely related opiates codeine and thebaine, and non-analgesic alkaloids such as papaverine and noscapine. The traditional, labor-intensive method of obtaining the latex is to scratch ("score") the immature seed pods (fruits) by hand; the latex leaks out and dries to a sticky yellowish residue that is later scraped off and dehydrated. The word "meconium" historically referred to related, weaker preparations made from other parts of the opium poppy or different species of poppies.

Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, Thēbai (Thebes), an ancient city in Upper Egypt. A minor constituent of opium, thebaine is chemically similar to both morphine and codeine, but has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)-thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine. While thebaine is not used therapeutically, it is the main alkaloid extracted from Papaver bracteatum and can be converted industrially into a variety of compounds, including hydrocodone, hydromorphone, oxycodone, oxymorphone, nalbuphine, naloxone, naltrexone, buprenorphine and etorphine. Butorphanol can also be derived from thebaine.

The term narcotic originally referred medically to any psychoactive compound with sleep-inducing properties, and euphoric properties as well. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine.

Opioids are substances that, when reaching opioid receptors, have effects similar to those of morphine. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, suppressing cough, as well as execution in the United States. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are frequently used non-medically for their euphoric effects or to prevent withdrawal.

The Papaveraceae are an economically important family of about 42 genera and approximately 775 known species of flowering plants in the order Ranunculales, informally known as the poppy family. The family is cosmopolitan, occurring in temperate and subtropical climates, but almost unknown in the tropics. Most are herbaceous plants, but a few are shrubs and small trees. The family currently includes two groups that have been considered to be separate families: Fumariaceae and Pteridophyllaceae.

Papaver somniferum, commonly known as the opium poppy or breadseed poppy, is a species of flowering plant in the family Papaveraceae. It is the species of plant from which both opium and poppy seeds are derived and is also a valuable ornamental plant, grown in gardens. Its native range is probably the eastern Mediterranean, but is now obscured by ancient introductions and cultivation, being naturalized across much of Europe and Asia.

Papaverine is an opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasm and vasospasm, and occasionally in the treatment of erectile dysfunction. It is used in the treatment of acute mesenteric ischemia. While it is found in the opium poppy, papaverine differs in both structure and pharmacological action from the analgesic morphine-like compounds.

Poppy tea is a herbal tea infusion brewed from poppy straw or seeds of several species of poppy. The species most commonly used for this purpose is Papaver somniferum, which produces opium as a natural defense against predators. In the live flower, opium is released when the surface of the bulb, called the seed pod, is pierced or scraped. For the purpose of the tea, dried pods are more commonly used than the pods of the live flower. The walls of the dried pods contain opiate alkaloids, primarily consisting of morphine.

Scoulerine, also known as discretamine and aequaline, is a benzylisoquinoline alkaloid (BIA) that is derived directly from (S)-reticuline through the action of berberine bridge enzyme. It is a precursor of other BIAs, notably berberine, noscapine, (S)-tetrahydropalmatine, and (S)-stylopine, as well as the alkaloids protopine, and sanguinarine. It is found in many plants, including opium poppy, Croton flavens, and certain plants in the genus Erythrina.
Pantopon, also known as Opium Alkaloids Hydrochlorides, is a preparation of opiates made up of all of the alkaloids present in opium in their natural proportions as hydrochlorides salts. It can sometimes be tolerated by people who are allergic to morphine.

Codeine is an opiate used to treat pain, coughing, and diarrhea. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children or adults. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours.

Metofoline (INN), also known as methofoline (USAN), is an opioid analgesic drug discovered in the 1950s by a team of Swiss researchers at Hoffmann-La Roche.
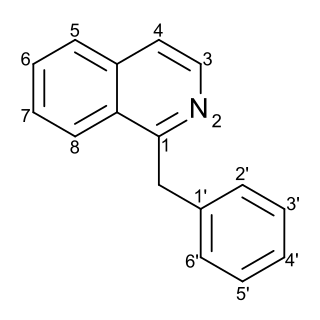
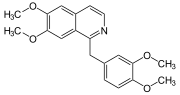
Substitution of the heterocycle isoquinoline at the C1 position by a benzyl group provides 1‑benzylisoquinoline, the most widely examined of the numerous benzylisoquinoline structural isomers. The 1-benzylisoquinoline moiety can be identified within numerous compounds of pharmaceutical interest, such as moxaverine; but most notably it is found within the structures of a wide variety of plant natural products, collectively referred to as benzylisoquinoline alkaloids. This class is exemplified in part by the following compounds: papaverine, noscapine, codeine, morphine, apomorphine, berberine, tubocurarine.

Poppy straw is derived from opium poppies that are harvested when fully mature and dried by mechanical means, minus the ripe poppy seeds. Opium poppy straw today can be one of several different things. It is what remains after the poppy seed harvest, that is, the dried stalks, stem and leaves of poppies grown for their seeds. The dried leaves and stalks are harvested after the seed pods have been used for traditional opium extraction. The field dried leaves, stalk and seed pod are used in commercial manufacture of morphine or other poppy alkaloid derived drugs, by first processing the material to make poppy straw separating the seeds then making concentrate of poppy straw, where no extraction using traditional methods of latex extraction has been made. The straw was originally considered an agricultural by-product of the mechanised poppy seed harvest, which was primarily grown for its edible and oil-producing seed. This changed in 1927 when János Kabay developed a chemical process to extract morphine from the crushed capsule. Concentrated poppy straw consisting mainly of the crushed capsule without the seeds soon became a valuable source of morphine. Today, concentrate of poppy straw is a major source of many opiates and other alkaloids. It is the source of 90% of the world supply of legal morphine and in some countries it also is a source of illegal morphine, which could be processed into illegal heroin.

Opiate is a term classically used in pharmacology to mean a drug derived from opium. Opioid, a more modern term, is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions with evidence of opiate trade and use for pain relief as early as the eighth century AD. Opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.
Spasmofen is the trade name for a combination drug used to relieve symptoms of painful cramps in smooth muscle, mainly in the bile ducts, urinary tract or gastrointestinal tract. The onset of relief can be felt after approximately 20 minutes, and last 3-5 hours.

Aporphine alkaloids are naturally occurring chemical compounds from the group of alkaloids. After the benzylisoquinoline alkaloids they are the second largest group of isoquinoline alkaloids.